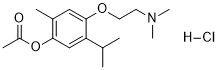
Moxisylyte hydrochloride is an alpha-adrenergic blocking agent that is used in Raynaud's disease. In order to reverse the mydriasis brought on by phenylephrine and other sympathomimetic drugs, it is also applied topically to the eye.
Physicochemical Properties
| Molecular Formula | C16H26CLNO3 |
| Molecular Weight | 315.84 |
| Exact Mass | 315.16 |
| Elemental Analysis | C, 60.85; H, 8.30; Cl, 11.22; N, 4.43; O, 15.20 |
| CAS # | 964-52-3 |
| Related CAS # | 964-52-3 |
| PubChem CID | 4260 |
| Appearance | White to off-white solid powder |
| Density | 1.018g/cm3 |
| Boiling Point | 371ºC at 760mmHg |
| Melting Point | 145ºC |
| Flash Point | 178.2ºC |
| LogP | 3.786 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 7 |
| Heavy Atom Count | 20 |
| Complexity | 304 |
| Defined Atom Stereocenter Count | 0 |
| SMILES | Cl.O=C(C)OC1C(C)=CC(OCCN(C)C)=C(C(C)C)C=1 |
| InChi Key | IPWGSXZCDPTDEH-UHFFFAOYSA-N |
| InChi Code | InChI=1S/C16H25NO3.ClH/c1-11(2)14-10-15(20-13(4)18)12(3)9-16(14)19-8-7-17(5)6;/h9-11H,7-8H2,1-6H3;1H |
| Chemical Name | [4-[2-(dimethylamino)ethoxy]-2-methyl-5-propan-2-ylphenyl] acetate;hydrochloride |
| Synonyms | Arlitene; Carlytene; Enfrental; Limatene; M-101; Moxilite; Enfrental; Limatene; M-101; Moxilite; Moxisylyte HCl; Moxisylyte hydrochloride; Opilon |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month Note: Please store this product in a sealed and protected environment, avoid exposure to moisture. |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| ADME/Pharmacokinetics |
Absorption, Distribution and Excretion Moxisylyte is rapidly absorbed after oral administration. Its pharmacokinetic profile is linear in the dose range from 10 to 30 mg for the values of Cmax and AUC. After intravenous administration, the maximal plasma concentration was of 352.8 ng/ml with an AUC of 152.6 mcg h/L. In preclinical trials, the bioavailability was always presented in approximately 10%. The major elimination route of moxisylyte is via the kidneys. The complete elimination of all the metabolites by urine is of 75% when administered intravenously and 69% when administered orally. From the elimination profile, The specific ranges of the two major metabolites of moxisylyte in the urine are of 50% and 10% for desacetyl-thymoxamine and N-monodemethyl-desacetyl-thymoxamine respectively. The fecal elimination corresponded only to the 14% of the administered dose. In preclinical trials, the volume of distribution presented for beagle dogs is in the range of 0.83-0.98 L/kg. In preclinical trials, the plasma clearance was of 7.17 ml min/kg for beagle dogs. Metabolism / Metabolites The pharmacokinetic profile of moxisylyte can make this drug to be considered as a prodrug as its biotransformation is very rapid. This drug gets rapidly hydrolyzed by pseudocholinesterase in plasma and tissues to give the major metabolite deacetyl-thymoxamine. This first metabolite is later demethylated by the cytochrome P450 monooxygenase system to form deacetyl-demethyl-thymoxamine. Both of this major metabolites are pharmacologically active. The pharmacokinetic studies with moxisylyte in urine and feces have shown the presence of 8 different metabolites, where two of them are highly polar and resistant to enzymatic hydrolysis. From this metabolites, it has been detected the sulfate and glucuronide conjugates of the major metabolites. Biological Half-Life The half-life of moxisylyte was of 1-2 hours. |
| References |
[1]. Are alpha-blockers involved in lower urinary tract dysfunction in multiple system atrophy? A comparison of prazosin and moxisylyte. 2000, March, 15, Pages 191-195. [2]. Moxisylyte: a review of its pharmacodynamic and pharmacokinetic properties, and its therapeutic use inimpotence. Fundam Clin Pharmacol, 1998, 12(4):377-87. |
| Additional Infomation |
Acetic acid [4-[2-(dimethylamino)ethoxy]-2-methyl-5-propan-2-ylphenyl] ester is a monoterpenoid. Moxisylyte, denominated as thymoxamine in the UK, is a specific and orally active α1-adrenergic antagonist. According to the WHO, moxisylyte is approved since 1987 and in the same year, it acquired the denomination of orphan product by the FDA. This drug was developed by the Japanese company Fujirebio and also by the American company Iolab in the late 80s. An alpha-adrenergic blocking agent that is used in Raynaud's disease. It is also used locally in the eye to reverse the mydriasis caused by phenylephrine and other sympathomimetic agents. (From Martindale, The Extra Pharmacopoeia, 30th ed, p1312) Drug Indication By the WHO, moxisylyte is indicated for the symptomatic management of sequelae of cerebral infarction or hemorrhage. The cerebral infarction is characterized by the blockage of the artery either by the formation of a thrombus or an embolus. On the other hand, the FDA classified moxisylyte for the reversal of phenylephrine-induced mydriasis in patients who have narrow anterior angles and are at risk of developing an acute attack of angle-closure glaucoma. Closed-angle glaucoma is caused by the contact between the iris and the trabecular meshwork. This contact will damage the aqueous outflow by the meshwork thus, increasing eye pressure and producing the symptoms of glaucoma. Mydriasis is referred to the dilatation of the pupils and this standard body function is known to be a trigger factor for the development of acute closed-angle glaucoma.This risk is explained by the generation of a pupillary block, which is the contact between the pupillary margins and the lens, thus preventing flow from the aqueous humor to the anterior chamber and followed by an increased pressure gradient. Moxisylyte is also approved in France as the first drug for the treatment of impotence. Mechanism of Action Moxisylyte is vasodilator that works as a specific alpha-adrenergic blocking agent. Its action is known to be competitive against norepinephrine without beta-receptor blocking, anti-angiotensin or anti-serotonin activity. Pharmacodynamics Administration of moxisylyte has shown to improve peripheral flow in occlusive arterial disease with little effect in blood pressure. There are reports of increases in cutaneous blood flow and skin temperature after local application of moxisylyte. |
Solubility Data
| Solubility (In Vitro) | DMSO: 20~63 mg/mL (63.3~199.5 mM) |
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2 mg/mL (6.33 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 20.0 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 2 mg/mL (6.33 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 20.0 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 3: ≥ 2 mg/mL (6.33 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 20.0 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. Solubility in Formulation 4: 100 mg/mL (316.62 mM) in PBS (add these co-solvents sequentially from left to right, and one by one), clear solution; with ultrasonication. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.1662 mL | 15.8308 mL | 31.6616 mL | |
| 5 mM | 0.6332 mL | 3.1662 mL | 6.3323 mL | |
| 10 mM | 0.3166 mL | 1.5831 mL | 3.1662 mL |