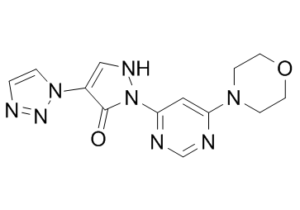
Molidustat (formerly known as BAY 85-3934) is a novel potent inhibitor of hypoxia-inducible factor prolyl hydroxylase (HIF-PH) which stimulates erythropoietin (EPO) production and the formation of red blood cells. The mean IC50 values of BAY 85-3934 for PHD1, PHD2, and PHD3 are 480 nM, 280 nM, and 450 nM, respectively. HIF stabilization by oral administration of the HIF-PH inhibitor BAY 85-3934 (molidustat) resulted in dose-dependent production of EPO in healthy Wistar rats and cynomolgus monkeys. In repeat oral dosing of BAY 85-3934, hemoglobin levels were increased compared with animals that received vehicle, while endogenous EPO remained within the normal physiological range. BAY 85-3934 therapy was also effective in the treatment of renal anemia in rats with impaired kidney function and, unlike treatment with rhEPO, resulted in normalization of hypertensive blood pressure in a rat model of CKD. Notably, unlike treatment with the antihypertensive enalapril, the blood pressure normalization was achieved without a compensatory activation of the renin-angiotensin system. Thus, BAY 85-3934 may provide an approach to the treatment of anemia in patients with CKD, without the increased risk of adverse cardiovascular effects seen for patients treated with rhEPO. Clinical studies are ongoing to investigate the effects of BAY 85-3934 therapy in patients with renal anemia.
Physicochemical Properties
| Molecular Formula | C13H14N8O2 | |
| Molecular Weight | 314.3 | |
| Exact Mass | 314.123 | |
| Elemental Analysis | C, 49.68; H, 4.49; N, 35.65; O, 10.18 | |
| CAS # | 1154028-82-6 | |
| Related CAS # | 1375799-59-9 (Sodium);1154028-82-6; | |
| PubChem CID | 59603622 | |
| Appearance | White to off-white solid powder | |
| Density | 1.7±0.1 g/cm3 | |
| Boiling Point | 589.2±60.0 °C at 760 mmHg | |
| Flash Point | 310.2±32.9 °C | |
| Vapour Pressure | 0.0±1.7 mmHg at 25°C | |
| Index of Refraction | 1.820 | |
| LogP | -1.77 | |
| Hydrogen Bond Donor Count | 1 | |
| Hydrogen Bond Acceptor Count | 8 | |
| Rotatable Bond Count | 3 | |
| Heavy Atom Count | 23 | |
| Complexity | 481 | |
| Defined Atom Stereocenter Count | 0 | |
| InChi Key | IJMBOKOTALXLKS-UHFFFAOYSA-N | |
| InChi Code | InChI=1S/C13H14N8O2/c22-13-10(20-2-1-16-18-20)8-17-21(13)12-7-11(14-9-15-12)19-3-5-23-6-4-19/h1-2,7-9,17H,3-6H2 | |
| Chemical Name | 2-(6-morpholin-4-ylpyrimidin-4-yl)-4-(triazol-1-yl)-1H-pyrazol-3-one | |
| Synonyms |
|
|
| HS Tariff Code | 2934.99.9001 | |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets |
PHD1 (IC50 = 480 nM); PHD2 (IC50 = 280 nM); PHD3 (IC50 =450 nM)[1]
Molidustat (BAY 85-3934) targets hypoxia-inducible factor prolyl hydroxylases (HIF-PHs, including PHD2); [1] Molidustat targets hypoxia-inducible factor prolyl hydroxylases (HIF-PHs); [2] |
||
| ln Vitro |
For PHD1, PHD2, and PHD3, the average IC50 values of BAY 85-3934 are 480 nM, 280 nM, and 450 nM, respectively. It only takes 20 minutes of exposure to 5 μM BAY 85-3934 for HeLa cells to produce detectable levels of HIF-1α. Using the hypoxia response element promoter as the control, BAY 85-3934 induced the expression of the firefly luciferase reporter gene in a cell reporter assay, with a mean (± SD) EC50 of 8.4±0.7 μM (n=4) [1]. 1. Molidustat (BAY 85-3934) inhibited hypoxia-inducible factor prolyl hydroxylase (PHD2) activity in concentration-dependent manners, and the inhibitory effect was modulated by the concentrations of 2-oxoglutarate, Fe²⁺, and ascorbate; the residual activity of PHD2 was measured with different concentrations of these cofactors and normalized to basal activity without the drug (100%) and residual activity (0%) [1] 2. Western blot analysis showed that Molidustat (BAY 85-3934) induced the expression of HIF-1α and HIF-2α in HeLa, A549, and Hep3B cells; in HeLa cells, HIF-1α was induced in a time-dependent manner after addition of serum-free medium containing 5 µM Molidustat (BAY 85-3934), while in A549 cells, HIF-1α induced by 20 µM Molidustat (BAY 85-3934) disappeared in a time-dependent manner after replacing the culture medium with medium containing cycloheximide (100 µM) [1] 3. In A549 HIF-RE2 reporter cells, Molidustat (BAY 85-3934) increased luciferase activity in a concentration-dependent manner, and the induction effect was affected by the presence of additional Fe²⁺; the luciferase activity was expressed as relative luciferase units (RLUs) [1] 4. Exposure of HeLa, A549, and Hep3B cells to Molidustat (BAY 85-3934) led to up-regulation of the mRNA expression levels of a panel of HIF target genes (fold-increase from baseline levels) [1] |
||
| ln Vivo |
When healthy Wistar rats and cynomolgus monkeys were given the HIF-PH inhibitor BAY 85-3934 (Molidustat) orally, it stabilized HIF and caused dose-dependent production of EPO. In addition to normalizing hypertensive blood pressure in a CKD rat model, molidustat therapy, in contrast to rhEPO therapy, is effective in managing renal anemia in rats with impaired renal function [1]. 1. In healthy male Wistar rats, single oral dosing of Molidustat (BAY 85-3934) resulted in a dose-dependent increase in plasma erythropoietin (EPO) levels at 4 h and reticulocyte proportion (as a percentage of red blood cells [RBCs]) at 72 h; data were pooled from two sequential experiments (n = 2×5 animals per group), with statistically significant differences compared with the vehicle group (p<0.05, p<0.01, p<0.001, unpaired t-test) [1] 2. Once-daily oral dosing of Molidustat (BAY 85-3934) in male Wistar rats increased packed cell volume (PCV) in a dose-dependent manner (n = 12 animals per group), with statistically significant differences compared with the vehicle group (p<0.01, p<0.001, two-way ANOVA with Dunnett’s multiple comparison test) [1] 3. In male Wistar rats, once-daily oral administration of Molidustat (BAY 85-3934) (2.5 mg/kg) induced erythropoiesis, and the effect was comparable to subcutaneous administration of recombinant human EPO (rhEPO) twice weekly (n = 10 animals per group), with a statistically significant increase in relevant erythropoietic parameters at day 30 (p<0.001, t-test) [1] 4. After oral administration of Molidustat (BAY 85-3934) (5 mg/kg) to male Wistar rats, the plasma drug levels, kidney EPO relative mRNA expression, and plasma EPO levels were measured (n = 5 animals per group); meanwhile, the mRNA expression levels of HIF target genes in rat kidney were up-regulated (baseline expression set at 1, n = 5 animals per group) [1] 5. In cynomolgus monkeys, repeat oral administration of Molidustat (BAY 85-3934) increased plasma EPO concentrations in a dose-dependent manner (n = 6 animals per group); a single oral dose of Molidustat (BAY 85-3934) (1.5 mg/kg) and a single subcutaneous dose of rhEPO (100 IU/kg) both increased plasma EPO concentrations (n = 3 animals per group); twice-weekly subcutaneous administration of rhEPO (100 IU/kg) for 2 weeks or once-daily oral administration of Molidustat (BAY 85-3934) (1.5 mg/kg) for 2 weeks both improved erythropoietic parameters (hemoglobin [HGB], red blood cells [RBCs], and reticulocytes) (n = 3 animals per group) [1] 6. In male Wistar rats with renal anemia induced by gentamicin, oral administration of Molidustat (BAY 85-3934) increased plasma EPO levels, and up-regulated EPO mRNA expression in kidney and liver at 4 h (n = 5 animals per group), with statistically significant differences compared with the vehicle group and control group (p<0.05, p<0.01, p<0.001, t-test); the mRNA expression levels of HIF target genes in kidney and liver were also up-regulated (n = 4 to 5 animals per group); once-daily oral dosing of Molidustat (BAY 85-3934) (five times per week) increased PCV and hemoglobin levels at day 22, with statistically significant differences compared with the vehicle group (p<0.05, p<0.01, p<0.001, t-test) [1] 7. In female Lewis rats with peptidoglycan-polysaccharide (PG-PS)-induced inflammatory anemia, oral administration of Molidustat (BAY 85-3934) increased PCV (n = 11–12 animals per group), and regulated the mRNA expression levels of EPO and monocyte chemotactic protein-1 (MCP-1) in kidney, and hepcidin mRNA in liver at the end of the study (p<0.05, p<0.01, p<0.001, t-test) [1] 8. In the rat subtotal nephrectomy model (CKD model), oral administration of Molidustat (BAY 85-3934) (2.5 mg/kg or 5 mg/kg once daily) for 5 weeks increased PCV, normalized systolic blood pressure (SBP), and did not cause compensatory activation of the renin-angiotensin system (prorenin levels were not abnormally elevated); compared with subcutaneous administration of rhEPO (100 IU/kg twice weekly) for 5 weeks, Molidustat (BAY 85-3934) did not induce hypertension (n = 4–6 animals per group); administration of Molidustat (BAY 85-3934) sodium (80 ppm), enalapril (30 ppm), or their combination in drinking water for 5 weeks also improved PCV, normalized SBP (at 4 weeks), and did not increase prorenin levels (n = 9–10 animals per group), with statistically significant differences compared with the sham or control group (p<0.05, p<0.01, p<0.001, one-way ANOVA followed by Dunnett’s/Bonferroni’s multiple comparison test) [1] 9. In a phase 3 clinical study involving Japanese nondialysis patients with renal anemia (CKD stages 3-5) previously treated with ESAs, Molidustat (initiated at 25 mg or 50 mg once daily, dose titrated to maintain Hb 11.0-13.0 g/dL) maintained mean Hb levels during the evaluation period (weeks 30-36) at 11.67 (95% CI: 11.48-11.85) g/dL, which was noninferior to darbepoetin alfa (initiated at a dose based on previous ESA dose, injected subcutaneously once every 2 or 4 weeks, dose titrated to maintain Hb 11.0-13.0 g/dL; mean Hb: 11.53 [95% CI: 11.31-11.74] g/dL); the least squares mean difference (molidustat-darbepoetin) in Hb change from baseline was 0.13 (95% CI: -0.15, 0.40) g/dL (noninferiority margin: 1.0 g/dL) [2] |
||
| Enzyme Assay |
Prolyl hydroxylase assay[1] The prolyl hydroxylase assay was performed as described previously with minor modifications. Biotinylated HIF-1α 556–574 (biotinyl-DLDLEMLAPYIPMDDDFQL) was bound to white 96-well NeutrAvidin high binding capacity plates, which were pre-blocked with Blocker Casein and subsequently blocked with 1 mM biotin. The immobilized peptide substrate was incubated with the appropriate amount of HIF-PH in buffer containing 20 mM Tris (pH 7.5), 5 mM KCl, 1.5 mM MgCl2, 20 µM 2-oxoglutarate, 10 µM FeSO4, 2 mM ascorbate, 4% protease inhibitors without EDTA in a final volume of 100 µl, with or without test compound added at appropriate concentrations. The reaction time was 60 min. To stop the reaction, plates were washed three times with wash buffer.[1] Hydroxylated biotinyl-HIF-1α 556–574 was incubated with Eu-VBC in 100 µl binding buffer (50 mM Tris [pH 7.5], 120 mM NaCl) for 60 min at room temperature. After washing six times with DELFIA wash buffer and adding 100 µl enhancer solution, the amount of bound VBC was determined by measuring time-resolved fluorescence with a Tecan infinite M200 plate reader. Measurements were taken in triplicate or more, and results were expressed as means ± SEM. IC50 values were determined after curve fitting using GraphPad Prism software applying the four-parameter logistic equation to the data sets. When adjustment of the concentration of free Fe2+ was necessary, the reaction buffer was supplemented with appropriate amounts of ammonium iron(II) sulfate ((NH4)2Fe(SO4)2.6H2O, Mohr’s salt). 1. To evaluate the inhibitory activity of Molidustat (BAY 85-3934) on PHD2, concentration-response curves of PHD2 activity were generated by adding increasing concentrations of 2-oxoglutarate, Fe²⁺, and ascorbate in the presence of Molidustat (BAY 85-3934); the activity data were presented as means ± SEM of 4 replicates, normalized to basal activity without the drug (100%) and residual activity (0%) [1] |
||
| Cell Assay |
Cell lines, cell culture media, and luciferase reporter assay[1] A549 and HeLa carcinoma cell lines (American Type Culture Collection) were cultured in DMEM/F-12, and Hep3B cells in RPMI medium, both supplemented with antibiotics, L-glutamine and 10% fetal calf serum. A549 cells stably transfected with the HIF-RE2-luc HIF reporter construct (constructed in pGL3) were seeded on 384-well plates at a density of 2500 cells/well in a volume of 25 µl complete cell culture medium, and re-incubated for 16–24 h before the test. Test compounds were added at appropriate dilutions in a volume of 10 µl, and cells were re-incubated for 6 h before measurement. Luciferase activity was determined in a luminometer after addition of cell lysis/luciferase buffer. Cell line identities were verified by STR DNA typing. Western blot analysis[1] For western blot analysis, cell lysates were separated on 4–12% SDS polyacrylamide gradient gels. Proteins were blotted onto polyvinylidene difluoride (PVDF) membranes. HIF-1α protein was detected using a HIF-1α specific monoclonal antibody at a dilution of 1∶250. HIF-2α protein was detected using a HIF-2α specific polyclonal antibody at a dilution of 1∶1000. Anti-β-actin antibody served as a loading control. Binding of the antibodies was visualized by binding of a horseradish peroxidase-conjugated anti-mouse IgG antibody, and subsequently enhanced using chemiluminescence, according to the manufactureŕs instructions. Novex Sharp Pre-stained Protein Standard was used as molecular weight marker. 1. Western blot analysis was performed to detect the expression of HIF-1α and HIF-2α in HeLa, A549, and Hep3B cells treated with Molidustat (BAY 85-3934); for the time-course experiment of HIF-1α induction in HeLa cells, serum-free medium containing 5 µM Molidustat (BAY 85-3934) was added, and HIF-1α levels were detected at different time points with β-actin as a loading control; for the time-course experiment of HIF-1α disappearance in A549 cells, cells were induced with 20 µM Molidustat (BAY 85-3934) first, then the culture medium was replaced with medium containing cycloheximide (100 µM), and HIF-1α levels were detected at different time points with β-actin as a loading control; all western blot experiments were repeated 3 times independently, and representative data were presented [1] 2. Luciferase activity assay was conducted in A549 HIF-RE2 reporter cells treated with Molidustat (BAY 85-3934) (with or without additional Fe²⁺); the luciferase activity (relative luciferase units [RLUs]) was measured, and data were presented as means ± SEM of 4 replicates [1] 3. Quantitative PCR was used to detect the relative mRNA expression levels of a panel of HIF target genes in HeLa, A549, and Hep3B cells exposed to Molidustat (BAY 85-3934); the mRNA levels were expressed as fold-increase from baseline levels, with data presented as means ± SD of 2 replicates [1] |
||
| Animal Protocol |
|
||
| ADME/Pharmacokinetics |
1. Plasma levels of Molidustat (BAY 85-3934) were measured in male Wistar rats after oral administration of 5 mg/kg of the drug (n = 5 animals per group) [1] |
||
| Toxicity/Toxicokinetics |
Overall, 94.5% of patients experienced at least 1 TEAE during the study: 92.7% of patients in the molidustat group and 96.3% in the darbepoetin group (Table 2). The most commonly reported TEAEs were nasopharyngitis (34.1% and 40.2% in the molidustat and darbepoetin groups, respectively), worsening of CKD (18.3% and 9.8%, respectively), and diarrhea (8.5% and 12.2%, respectively) (Table 2). TEAEs leading to death were reported in 2 patients (2.4%) in the molidustat group and none in the darbepoetin group, and serious TEAEs were reported in 32.9% and 26.8% of patients, respectively. MACEs that occurred after the start of the study drug were reported in 3.7% of patients treated with molidustat and 1.2% of patients receiving darbepoetin (online suppl. Table 3). Additionally, 3.7% of patients in the molidustat group and 1.2% in the darbepoetin group developed diabetic retinopathy, and 3.7% in the molidustat group and 4.9% in the darbepoetin group developed neoplasms (benign, malignant, or unspecified) (online suppl. Table 4). The mean serum eGFR appeared to remain stable in the molidustat group (online suppl. Fig. 7). Subgroup analyses of TEAEs by age group (<65 and ≥65 years old) and by sex are presented in online supplementary Table 5. The proportion of serious TEAEs was similar between the 2 groups in female patients but higher for males in the molidustat group than in the darbepoetin group.[2] 1. In the phase 3 clinical study, 92.7% of patients in the Molidustat group reported at least 1 treatment-emergent adverse event (TEAE), compared with 96.3% in the darbepoetin group; TEAEs leading to death were reported in 2 patients (2.4%) in the Molidustat group and none in the darbepoetin group; serious TEAEs were reported in 32.9% of patients in the Molidustat group and 26.8% in the darbepoetin group; no new safety signal was observed for Molidustat [2] |
||
| References |
[1]. Mimicking hypoxia to treat anemia: HIF-stabilizer BAY 85-3934 (Molidustat) stimulates erythropoietin production without hypertensive effects. PLoS One. 2014 Nov 13;9(11):e111838. [2]. Molidustat for Renal Anemia in Nondialysis Patients Previously Treated with Erythropoiesis-Stimulating Agents: A Randomized, Open-Label, Phase 3 Study. Am J Nephrol. 2021;52(10-11):884-893. |
||
| Additional Infomation |
Molidustat is under investigation in clinical trial NCT03350321 (A Study of Molidustat for Correction of Renal Anemia in Non-dialysis Subjects). See also: Molidustat Sodium (active moiety of). 1. Oxygen sensing by HIF-PHs is the dominant regulatory mechanism of EPO expression; in chronic kidney disease (CKD), impaired EPO expression causes anemia, and rhEPO supplementation may lead to excessive EPO levels and hypertension; inhibiting HIF-PHs to stabilize HIF is a novel strategy to restore endogenous EPO production [1] 2. Molidustat (BAY 85-3934) is an oral HIF-PH inhibitor that can stimulate endogenous EPO production within the normal physiological range, increase hemoglobin levels, and normalize hypertensive blood pressure in CKD rat models without compensatory activation of the renin-angiotensin system [1] 3. Molidustat (BAY 85-3934) is effective in treating renal anemia in rats with impaired kidney function and inflammatory anemia in rats, and has potential for treating anemia in CKD patients without the cardiovascular risks associated with rhEPO [1] 4. Molidustat is an oral hypoxia-inducible factor prolyl hydroxylase inhibitor investigated as an alternative treatment for renal anemia; the MIYABI program includes five phase 3 studies to evaluate the efficacy and safety of Molidustat [2] 5. In Japanese nondialysis CKD patients with renal anemia previously treated with ESAs, Molidustat was noninferior to darbepoetin alfa in maintaining Hb levels within the target range (11.0-13.0 g/dL) and was well tolerated [2] |
Solubility Data
| Solubility (In Vitro) |
|
|||
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 0.5 mg/mL (1.59 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 5.0 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 0.5 mg/mL (1.59 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 5.0 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 3: ≥ 0.5 mg/mL (1.59 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 5.0 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. Solubility in Formulation 4: 10 mg/mL (31.82 mM) in 0.5% CMC-Na/saline water (add these co-solvents sequentially from left to right, and one by one), suspension solution; with ultrasonication. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.1817 mL | 15.9084 mL | 31.8167 mL | |
| 5 mM | 0.6363 mL | 3.1817 mL | 6.3633 mL | |
| 10 mM | 0.3182 mL | 1.5908 mL | 3.1817 mL |