Physicochemical Properties
| Molecular Formula | C7H12BRNO2 |
| Molecular Weight | 222.08 |
| Exact Mass | 221.005 |
| CAS # | 17210-51-4 |
| Related CAS # | Guvacoline hydrochloride;6197-39-3 |
| PubChem CID | 15560296 |
| Appearance | Typically exists as solid at room temperature |
| LogP | 1.366 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 2 |
| Heavy Atom Count | 11 |
| Complexity | 163 |
| Defined Atom Stereocenter Count | 0 |
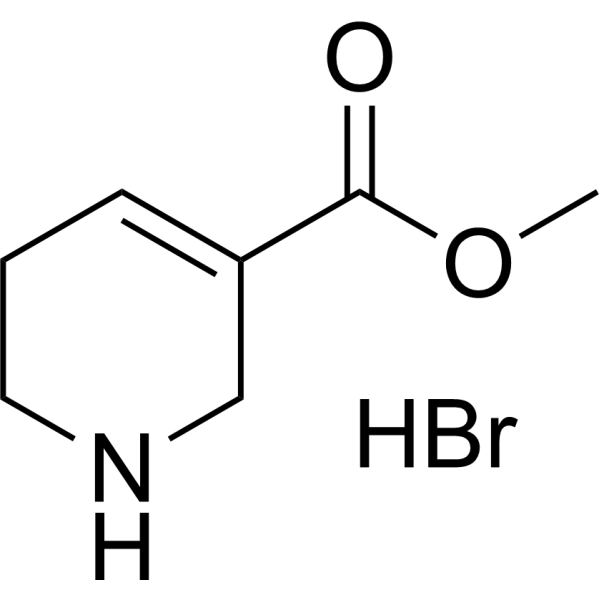
| SMILES | COC(=O)C1=CCCNC1.Br |
| InChi Key | DOXOETUWVNVADB-UHFFFAOYSA-N |
| InChi Code | InChI=1S/C7H11NO2.BrH/c1-10-7(9)6-3-2-4-8-5-6;/h3,8H,2,4-5H2,1H3;1H |
| Chemical Name | methyl 1,2,3,6-tetrahydropyridine-5-carboxylate;hydrobromide |
| Synonyms | Guvacoline Hydrobromide; 17210-51-4; Guvacoline (hydrobromide); Guvacoline, Hydrobromide; methyl 1,2,3,6-tetrahydropyridine-5-carboxylate;hydrobromide; Guvacoline (hydrobromide?); SCHEMBL8872254; DTXSID20574157; |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | AChR; natural pyridine alkaloid from Areca triandra |
| ln Vitro | A series of tertiary and quaternary N-substituted guvacine (1,2,5,6-tetrahydro-3-carboxy-pyridine) methyl and propargyl esters have been synthesized and tested for muscarinic/antimuscarinic activity on rat ileum and electrically paced left atria. Arecoline and arecaidine propargyl ester (APE) as well as their corresponding N-demethyl derivatives, Guvacoline (norarecoline) and guvacine propargyl ester, acted as full agonists at both atrial and ileal muscarinic receptors (range of pD2-values 6.09-8.07). However, in both preparations arecoline and APE were clearly more potent (up to 15-fold) than their N-demethyl analogues. Replacement of the N-methyl group in arecoline and APE by larger substituents (ethyl, n-propyl, n-butyl, benzyl, phenylethyl) as well as N-methylation resulted in a decrease or even a complete loss of agonistic activity. In both organs, the propargyl esters usually showed higher potency than the corresponding methyl ester analogues. N-Ethylguvacine propargyl ester and APE methiodide displayed pronounced agonistic activity in the atria (pD2 approximately 6.5; intrinsic activity = 0.79 and 0.67, respectively) but behaved as competitive antagonists in the ileum (pA2 = 6.06 and 5.62, respectively). Beside the lower sensitivity to muscarinic agonists of the rat ileum as compared to rat atria, the cardioselective stimulant action of both agents may also be due to their ability to recognize structural differences between atrial M2 alpha and ileal M2 beta muscarinic receptor subtypes [2]. |
| ln Vivo | Areca nuts (seeds of Areca catechu L.) are a traditional and popular masticatory in India, Bangladesh, Malaysia, certain parts of China, and some other countries. Four related pyridine alkaloids (arecoline, arecaidine, Guvacoline, and guvacine) are considered being the main functional ingredients in areca nut. Until now, A. catechu is the only known species producing these alkaloids in the Arecaceae family. In the present study, we investigated alkaloid contents in 12 Arecaceae species and found that only Areca triandra Roxb. contained these pyridine alkaloids. We further analyzed in more detail tissue-specific and development-related distribution of these alkaloids in leaves, male and female flowers and fruits in different stages of maturity in A. triandra by ultra-performance liquid chromatography-quadrupole/time-of-flight mass spectrometry. Results revealed that the alkaloids were most abundant in young leaves, the pericarp of ripe fruits and the endosperm of unripe fruits in developmental stage 2. Abundance of the 4 different alkaloids in A. triandra fruits varied during maturation. Pericarps of ripe fruits had the highest arecaidine concentration (4.45 mg g-1) and the lowest Guvacoline concentration (0.0175 mg g-1), whereas the endosperm of unripe fruits of developmental stage 2 contained the highest Guvacoline concentration (3.39 mg g-1) and the lowest guvacine concentration (0.245 mg g-1). We conclude that A. triandra is useful in future as a further valuable source of Areca alkaloids [1]. |
| Enzyme Assay |
Alkaloid extraction from A. triandra [1] A modified method for the extraction of alkaloids in A. triandra tissue was used (Pan et al. 2018). Samples (100 mg dry weight) were lypophilized, homogenized in a ball mill and extracted with 1.2 mL of 50% methanol containing 0.1% formic acid. The homogenate was vortexed for 2 min, sonicated for 35 min and was kept at − 20 °C for 3–5 h. The mixture was vortexed again for 3 min at room temperature, centrifuged at 10 000g for 15 min at room temperature using a refrigerated centrifuge. The supernatant was transferred to a new vial and diluted 1000 times. Samples were stored at − 20 °C until analysis. 10 μL of the samples were analyzed by UPLC-MS. Alkaloid standards were dissolved in 95% acetonitrile containing 5 mM ammonium, giving stock solutions with concentrations of 1 μg μL−1. Stock solutions were stepwise diluted with 95% acetonitrile containing 5 mM ammonium formate, giving final concentrations of 1, 2, 4, 8 and 16 pg μL−1 for arecoline and arecaidine; 40, 80, 200, 400 and 800 pg μL−1 for Guvacoline; and 100, 200, 400, 800 and 1600 pg μL−1 for guvacine, respectively. |
| References |
[1]. Tissue-specific and maturity-dependent distribution of pyridine alkaloids in Areca triandra. J Plant Res. 2019 Jul;132(4):531-540. [2]. Synthesis and muscarinic activity of a series of tertiary and quaternary N-substituted guvacine esters structurally related to arecoline and arecaidine propargyl ester. Arzneimittelforschung. 1989 May;39(5):539-44. |
| Additional Infomation | We were also interested in the alkaloid concentration and distribution in other plant parts. In flowers, the highest alkaloid level can be found in female flowers (1.26 mg g−1) and the lowest alkaloid concentration was detected in male flowers (0.624 mg g−1) (Fig. 4c). Guvacoline was the major alkaloid in flowers. Spadices, female flowers and male flowers contained 0.697, 0.932 and 0.360 mg g−1 Guvacoline, respectively. Arecaidine (2.74 mg g−1) was the major alkaloid in tender leaves, while Guvacoline (0.612 mg g−1) was the major alkaloid in ripe leaves (Fig. 4d). Total alkaloid contents were 5.21 times higher in tender leaves than in ripe leaves. [1] |
Solubility Data
| Solubility (In Vitro) | May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples |
| Solubility (In Vivo) |
Note: Listed below are some common formulations that may be used to formulate products with low water solubility (e.g. < 1 mg/mL), you may test these formulations using a minute amount of products to avoid loss of samples. Injection Formulations (e.g. IP/IV/IM/SC) Injection Formulation 1: DMSO : Tween 80: Saline = 10 : 5 : 85 (i.e. 100 μL DMSO stock solution → 50 μL Tween 80 → 850 μL Saline) *Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH ₂ O to obtain a clear solution. Injection Formulation 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL DMSO → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Injection Formulation 3: DMSO : Corn oil = 10 : 90 (i.e. 100 μL DMSO → 900 μL Corn oil) Example: Take the Injection Formulation 3 (DMSO : Corn oil = 10 : 90) as an example, if 1 mL of 2.5 mg/mL working solution is to be prepared, you can take 100 μL 25 mg/mL DMSO stock solution and add to 900 μL corn oil, mix well to obtain a clear or suspension solution (2.5 mg/mL, ready for use in animals). Injection Formulation 4: DMSO : 20% SBE-β-CD in saline = 10 : 90 [i.e. 100 μL DMSO → 900 μL (20% SBE-β-CD in saline)] *Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Injection Formulation 5: 2-Hydroxypropyl-β-cyclodextrin : Saline = 50 : 50 (i.e. 500 μL 2-Hydroxypropyl-β-cyclodextrin → 500 μL Saline) Injection Formulation 6: DMSO : PEG300 : castor oil : Saline = 5 : 10 : 20 : 65 (i.e. 50 μL DMSO → 100 μLPEG300 → 200 μL castor oil → 650 μL Saline) Injection Formulation 7: Ethanol : Cremophor : Saline = 10: 10 : 80 (i.e. 100 μL Ethanol → 100 μL Cremophor → 800 μL Saline) Injection Formulation 8: Dissolve in Cremophor/Ethanol (50 : 50), then diluted by Saline Injection Formulation 9: EtOH : Corn oil = 10 : 90 (i.e. 100 μL EtOH → 900 μL Corn oil) Injection Formulation 10: EtOH : PEG300:Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL EtOH → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Oral Formulations Oral Formulation 1: Suspend in 0.5% CMC Na (carboxymethylcellulose sodium) Oral Formulation 2: Suspend in 0.5% Carboxymethyl cellulose Example: Take the Oral Formulation 1 (Suspend in 0.5% CMC Na) as an example, if 100 mL of 2.5 mg/mL working solution is to be prepared, you can first prepare 0.5% CMC Na solution by measuring 0.5 g CMC Na and dissolve it in 100 mL ddH2O to obtain a clear solution; then add 250 mg of the product to 100 mL 0.5% CMC Na solution, to make the suspension solution (2.5 mg/mL, ready for use in animals). Oral Formulation 3: Dissolved in PEG400 Oral Formulation 4: Suspend in 0.2% Carboxymethyl cellulose Oral Formulation 5: Dissolve in 0.25% Tween 80 and 0.5% Carboxymethyl cellulose Oral Formulation 6: Mixing with food powders Note: Please be aware that the above formulations are for reference only. InvivoChem strongly recommends customers to read literature methods/protocols carefully before determining which formulation you should use for in vivo studies, as different compounds have different solubility properties and have to be formulated differently. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 4.5029 mL | 22.5144 mL | 45.0288 mL | |
| 5 mM | 0.9006 mL | 4.5029 mL | 9.0058 mL | |
| 10 mM | 0.4503 mL | 2.2514 mL | 4.5029 mL |