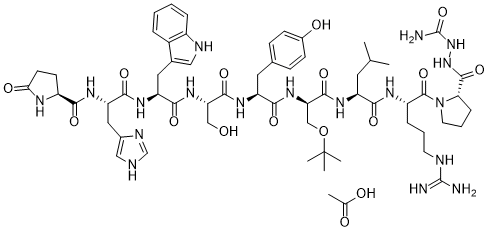
Goserelin is a synthetic decapeptide analogue of luteinizing hormone-releasing hormone (LHRH) with antineoplastic activity. Goserelin binds to gonadotropin-releasing hormone (GnRH) receptors in the pituitary and activates them. Goserelin inhibits pituitary gonadotropin secretion, which lowers testosterone (in males) and estradiol (in females) levels when taken for an extended period of time. Regression of sex hormone-sensitive tumors and reduction of sex organ size and function may occur from the administration of this agent in a depot formulation.
Physicochemical Properties
| Molecular Formula | C61H88N18O16 |
| Molecular Weight | 1329.485 |
| Exact Mass | 1328.66 |
| Elemental Analysis | C, 55.11; H, 6.67; N, 18.96; O, 19.25 |
| CAS # | 145781-92-6 |
| Related CAS # | Goserelin; 65807-02-5 |
| PubChem CID | 16052011 |
| Appearance | White to off-white solid powder |
| Density | 1.5±0.1 g/cm3 |
| Boiling Point | 1695.5ºC at 760mmHg |
| Melting Point | >190°C (dec.) |
| Vapour Pressure | 0mmHg at 25°C |
| Index of Refraction | 1.692 |
| LogP | -0.95 |
| Hydrogen Bond Donor Count | 18 |
| Hydrogen Bond Acceptor Count | 18 |
| Rotatable Bond Count | 32 |
| Heavy Atom Count | 95 |
| Complexity | 2590 |
| Defined Atom Stereocenter Count | 9 |
| SMILES | O=C(N[C@H](C(N[C@@H](CC1=CC=C(O)C=C1)C(N[C@H](COC(C)(C)C)C(N[C@@H](CC(C)C)C(N[C@@H](CCCNC(N)=N)C(N2CCC[C@H]2C(NNC(N)=O)=O)=O)=O)=O)=O)=O)CO)[C@@H](NC([C@H](CC3=CN=CN3)NC([C@@H](N4)CCC4=O)=O)=O)CC5=CNC6=C5C=CC=C6.CC(O)=O |
| InChi Key | IKDXDQDKCZPQSZ-JHYYTBFNSA-N |
| InChi Code | InChI=1S/C59H84N18O14.C2H4O2/c1-31(2)22-40(49(82)68-39(12-8-20-64-57(60)61)56(89)77-21-9-13-46(77)55(88)75-76-58(62)90)69-54(87)45(29-91-59(3,4)5)74-50(83)41(23-32-14-16-35(79)17-15-32)70-53(86)44(28-78)73-51(84)42(24-33-26-65-37-11-7-6-10-36(33)37)71-52(85)43(25-34-27-63-30-66-34)72-48(81)38-18-19-47(80)67-38;1-2(3)4/h6-7,10-11,14-17,26-27,30-31,38-46,65,78-79H,8-9,12-13,18-25,28-29H2,1-5H3,(H,63,66)(H,67,80)(H,68,82)(H,69,87)(H,70,86)(H,71,85)(H,72,81)(H,73,84)(H,74,83)(H,75,88)(H4,60,61,64)(H3,62,76,90);1H3,(H,3,4)/t38-,39-,40-,41-,42-,43-,44-,45+,46-;/m0./s1 |
| Chemical Name | acetic acid;(2S)-N-[(2S)-1-[[(2S)-1-[[(2S)-1-[[(2S)-1-[[(2R)-1-[[(2S)-1-[[(2S)-1-[(2S)-2-[(carbamoylamino)carbamoyl]pyrrolidin-1-yl]-5-(diaminomethylideneamino)-1-oxopentan-2-yl]amino]-4-methyl-1-oxopentan-2-yl]amino]-3-[(2-methylpropan-2-yl)oxy]-1-oxopropan-2-yl]amino]-3-(4-hydroxyphenyl)-1-oxopropan-2-yl]amino]-3-hydroxy-1-oxopropan-2-yl]amino]-3-(1H-indol-3-yl)-1-oxopropan-2-yl]amino]-3-(1H-imidazol-5-yl)-1-oxopropan-2-yl]-5-oxopyrrolidine-2-carboxamide |
| Synonyms | Goserelin Acetate; ICI-118630; ICI118630; ICI 118630; brand name: Zoladex |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month Note: Please store this product in a sealed and protected environment, avoid exposure to moisture. |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | GnRH |
| ln Vitro | Goserelin (1 nM-1 mM; 48-72 hours) promotes expression in EOC cells [1]. Goserelin (100 μM; 24-72 hours) regulates the expression of human liver cancer-related genes in SKOV3-ip cells [1]. (100 μM; 24-72 hours) promotes EOC cell inflammation by upregulating FOXO1 through PI3K/AKT signaling [1]. Apoptosis analysis [1] Cell Line: SKOV3 cells, SKOV3-ip cells, A2780 cells (human EOC cell line) Concentration: 1 nM, 10 nM, 100 nM, 1 μM, 10 μM, 100 μM, 1 mM Incubation time : 48 hours, 72 hours results: significantly increased the total apoptosis rate of SKOV3-ip, SKOV3 and A2780 cells. Western Blot Analysis[1] Cell lines: SKOV3 cells, SKOV3-ip cells, A2780 cells (human EOC cell line) Concentration: 1 nM, 10 nM, 100 nM, 1 μM, 10 μM, 100 μM, 1 mM Incubation time: Results at 48 hours and 72 hours: At 100 μM, the expression of cleaved-caspase-3 and cleaved-PARP increased significantly. RT-PCR[1] Cell line: SKOV3-ip Cell concentration: 100 μM Incubation time: 24 hours, 48 hours, 72 hours Results: Expression of human apoptosis-related genes is regulated |
| ln Vivo | Goserelin acetate (100 μg; subcutaneous injection; daily; for 19 days) significantly increases the proportion of anode cells in subcutaneous xenograft tumors [1]. Animal model: Five-week-old specific pathogen-free (SPF) female nude mice (18-20 g), subcutaneous xenograft tumor model [1] Dosage: 100 µg/mouse Administration: subcutaneous injection, daily, for 19 days Results : Significantly increases the proportion of apoptotic cells in subcutaneous xenograft tumors |
| Cell Assay |
Cell Line: SKOV3 cells, SKOV3-ip cells, A2780 cells (human EOC cell lines) Concentration: 1 nM, 10 nM, 100 nM, 1 μM, 10 μM, 100 μM, 1 mM Incubation Time: 48 hours, 72 hours Result: Significantly increased the total apoptosis rate of SKOV3-ip, SKOV3 and A2780 cells |
| Animal Protocol |
Five-week-old specific-pathogen free (SPF) female nude mice (18-20 g), subcutaneous xenograft tumor model 100 µg/mice Subcutaneous injection, daily, for 19 days |
| References |
[1]. Goserelin promotes the apoptosis of epithelial ovarian cancer cells by upregulating forkhead box O1 through the PI3K/AKT signaling pathway. Oncol Rep. 2018 Mar; 39(3): 1034–1042. [2]. Goserelin for ovarian protection during breast-cancer adjuvant chemotherapy. N Engl J Med. 2015 Mar 5;372(10):923-32. |
| Additional Infomation |
Goserelin Acetate can cause developmental toxicity, female reproductive toxicity and male reproductive toxicity according to state or federal government labeling requirements. Goserelin Acetate is the acetate salt of a synthetic decapeptide analog of luteinizing hormone-releasing hormone (LHRH). Continuous, prolonged administration of goserelin in males results in inhibition of pituitary gonadotropin secretion, leading to a significant decline in testosterone production; in females, prolonged administration results in a decrease in estradiol production. (NCI04) A synthetic long-acting agonist of GONADOTROPIN-RELEASING HORMONE. Goserelin is used in treatments of malignant NEOPLASMS of the prostate, uterine fibromas, and metastatic breast cancer. See also: Goserelin (has active moiety). |
Solubility Data
| Solubility (In Vitro) |
H2O: ~100 mg/mL (~75.2 mM) DMSO: ≥ 28 mg/mL (~21.1 mM) |
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.08 mg/mL (1.56 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 20.8 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 2.08 mg/mL (1.56 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 20.8 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 3: 2.08 mg/mL (1.56 mM) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 20.8 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. Solubility in Formulation 4: 50 mg/mL (37.61 mM) in PBS (add these co-solvents sequentially from left to right, and one by one), clear solution; with ultrasonication. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 0.7522 mL | 3.7608 mL | 7.5217 mL | |
| 5 mM | 0.1504 mL | 0.7522 mL | 1.5043 mL | |
| 10 mM | 0.0752 mL | 0.3761 mL | 0.7522 mL |