Physicochemical Properties
| Molecular Formula | C84H107CLN18O18 |
| Molecular Weight | 1692.31 |
| Exact Mass | 1708.78 |
| Elemental Analysis | C, 60.34; H, 6.36; Cl, 2.17; N, 15.45; O, 15.68 |
| CAS # | 934016-19-0 |
| Related CAS # | Degarelix; 214766-78-6;Degarelix-d7;934016-19-0;934246-14-7 |
| PubChem CID | 16186010 |
| Appearance | White to off-white solid powder |
| Hydrogen Bond Donor Count | 18 |
| Hydrogen Bond Acceptor Count | 20 |
| Rotatable Bond Count | 41 |
| Heavy Atom Count | 121 |
| Complexity | 3420 |
| Defined Atom Stereocenter Count | 11 |
| SMILES | ClC1C=CC(=CC=1)C[C@H](C(N[C@H](CC1C=NC=CC=1)C(N[C@@H](CO)C(N[C@@H](CC1C=CC(=CC=1)NC([C@@H]1CC(NC(N1)=O)=O)=O)C(N[C@H](CC1C=CC(=CC=1)NC(N)=O)C(N[C@@H](CC(C)C)C(N[C@@H](CCCCNC(C)C)C(N1CCC[C@H]1C(N[C@@H](C(N)=O)C)=O)=O)=O)=O)=O)=O)=O)=O)NC([C@@H](CC1C=CC2C=CC=CC=2C=1)NC(C)=O)=O.OC(C)=O |
| InChi Key | QMBXFMRFTMPFEY-YECCWIQASA-N |
| InChi Code | InChI=1S/C82H103ClN18O16.C2H4O2.H2O/c1-45(2)35-60(72(107)92-59(16-9-10-33-87-46(3)4)80(115)101-34-12-17-68(101)79(114)88-47(5)70(84)105)93-74(109)63(38-51-23-30-58(31-24-51)91-81(85)116)95-76(111)64(39-50-21-28-57(29-22-50)90-71(106)66-42-69(104)100-82(117)99-66)97-78(113)67(44-102)98-77(112)65(41-53-13-11-32-86-43-53)96-75(110)62(37-49-19-26-56(83)27-20-49)94-73(108)61(89-48(6)103)40-52-18-25-54-14-7-8-15-55(54)36-52;1-2(3)4;/h7-8,11,13-15,18-32,36,43,45-47,59-68,87,102H,9-10,12,16-17,33-35,37-42,44H2,1-6H3,(H2,84,105)(H,88,114)(H,89,103)(H,90,106)(H,92,107)(H,93,109)(H,94,108)(H,95,111)(H,96,110)(H,97,113)(H,98,112)(H3,85,91,116)(H2,99,100,104,117);1H3,(H,3,4);1H2/t47-,59+,60+,61-,62-,63-,64+,65-,66+,67+,68+;;/m1../s1 |
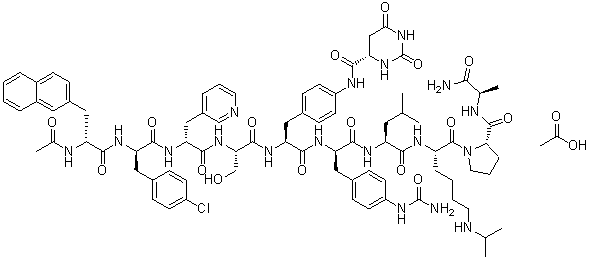
| Chemical Name | (4S)-N-[4-[(2S)-2-[[(2S)-2-[[(2R)-2-[[(2R)-2-[[(2R)-2-acetamido-3-naphthalen-2-ylpropanoyl]amino]-3-(4-chlorophenyl)propanoyl]amino]-3-pyridin-3-ylpropanoyl]amino]-3-hydroxypropanoyl]amino]-3-[[(2R)-1-[[(2S)-1-[[(2S)-1-[(2S)-2-[[(2R)-1-amino-1-oxopropan-2-yl]carbamoyl]pyrrolidin-1-yl]-1-oxo-6-(propan-2-ylamino)hexan-2-yl]amino]-4-methyl-1-oxopentan-2-yl]amino]-3-[4-(carbamoylamino)phenyl]-1-oxopropan-2-yl]amino]-3-oxopropyl]phenyl]-2,6-dioxo-1,3-diazinane-4-carboxamide;acetic acid;hydrate |
| Synonyms | FE 200486; FE200486; FE-200486; ASP-3550; ASP 3550; ASP3550; Degarelix acetate; tradename Firmagon |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| ln Vitro | Degarelix exhibits only very weak histamine-releasing properties and has the lowest histamine-releasing capacity among LHRH antagonists, including Cetrorelix, Abarelix, and Ganirelix [1]. Degarelix (1 nM-10 μM, 0-72 hours) reduces cell viability in all prostate cell lines (WPE1-NA22, WPMY-1, BPH-1, VCaP cells), except PC-3 cells [2] . Degarelix (10 μM, 0-72 hours) exerts a direct effect on prostate cell growth through apoptosis [2]. Cell viability assay[2] Cell lines: WPMY-1, WPE1-NA22, BPH-1, LNCaP and VCaP Concentration: 1 nM-10 μM Incubation time: 48 hours and 72 hours for WPMY-1 cells, 72 hours for WPE1-NA22 cells , BPH-1 cells (48 hours and 72 hours), LNCaP cells (48 hours and 72 hours) Results: Cell viability was reduced in all prostate cell lines except PC-3 cells. Apoptosis analysis[2] Cell lines: WPE1-NA22, BPH-1, LNCaP and VCaP Concentration: 10 μM Incubation time: 24, 48 and 72 hours Results: Induced significant increase in caspase 3/7 activation. |
| ln Vivo | Degarelix (0-10 μg/kg; subcutaneous injection; once) reduces plasma LH levels and plasma testosterone levels in castrated rats in a dose-dependent manner [3]. Degarelix is stable when incubated in microsomes and cryopreserved hepatocytes from animal liver tissue. In rats and dogs, the majority of the degarelix dose is eliminated via urine and feces in equal amounts (40-50% in each matrix) within 48 hours, whereas in monkeys the main route of excretion is feces (50 %) and kidney (22%)[4]. Animal model: Male Sprague-Dawley rat, castrated [3] Dosage: 0.3, 1, 3 and 10 μg/kg or 12.5, 50 and 200 μg/kg Administration: subcutaneous injection, once Result: Dose-dependent And the minimum reversible effective dose is 3 μg/kg to reduce plasma LH levels. For the 50 μg/kg and 200 μg/kg doses, absorption t1/2 values were 4 minutes and 30 minutes, Tmax values were 1 hour and 5 hours, and apparent plasma disappearance t1/2 values were 12 hours and 67 hours, respectively. The minimum effective dose is 1 μg/kg, and plasma testosterone levels decrease in a dose-dependent manner. |
| References |
[1]. An update on the use of degarelix in the treatment of advanced hormone-dependent prostate cancer. Onco Targets Ther. 2013 Apr 16;6:391-402. [2]. In search of the molecular mechanisms mediating the inhibitory effect of the GnRH antagonistdegarelix on human prostate cell growth. PLoS One. 2015 Mar 26;10(3):e0120670. [3]. Pharmacological profile of a new, potent, and long-acting gonadotropin-releasing hormoneantagonist: degarelix. J Pharmacol Exp Ther. 2002 Apr;301(1):95-102. [4]. Metabolite profiles of degarelix, a new gonadotropin-releasing hormone receptor antagonist, in rat, dog, and monkey. Drug Metab Dispos. 2011 Oct;39(10):1895-903. |
| Additional Infomation |
Degarelix Acetate is the acetate form of a long-acting, synthetic peptide with gonadotrophin-releasing hormone (GnRH) antagonistic properties. Degarelix targets and blocks GnRH receptors located on the surfaces of gonadotroph cells in the anterior pituitary, thereby reducing secretion of luteinizing hormone (LH) by pituitary gonadotroph cells and so decreasing testosterone production by interstitial (Leydig) cells in the testes. See also: Degarelix (has active moiety). Drug Indication Degarelix Accord is a gonadotrophin releasing hormone (GnRH) antagonist indicated: for treatment of adult male patients with advanced hormone-dependent prostate cancer . for treatment of high-risk localised and locally advanced hormone dependent prostate cancer in combination with radiotherapy. as neo-adjuvant treatment prior to radiotherapy in patients with high-risk localised or locally advanced hormone dependent prostate cancer . FIRMAGON is a gonadotrophin releasing hormone (GnRH) antagonist indicated: - for treatment of adult male patients with advanced hormone-dependent prostate cancer . - for treatment of high-risk localised and locally advanced hormone dependent prostate cancer in combination with radiotherapy. - as neo-adjuvant treatment prior to radiotherapy in patients with high-risk localised or locally advanced hormone dependent prostate cancer . |
Solubility Data
| Solubility (In Vitro) | May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples |
| Solubility (In Vivo) |
Note: Listed below are some common formulations that may be used to formulate products with low water solubility (e.g. < 1 mg/mL), you may test these formulations using a minute amount of products to avoid loss of samples. Injection Formulations (e.g. IP/IV/IM/SC) Injection Formulation 1: DMSO : Tween 80: Saline = 10 : 5 : 85 (i.e. 100 μL DMSO stock solution → 50 μL Tween 80 → 850 μL Saline) *Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH ₂ O to obtain a clear solution. Injection Formulation 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL DMSO → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Injection Formulation 3: DMSO : Corn oil = 10 : 90 (i.e. 100 μL DMSO → 900 μL Corn oil) Example: Take the Injection Formulation 3 (DMSO : Corn oil = 10 : 90) as an example, if 1 mL of 2.5 mg/mL working solution is to be prepared, you can take 100 μL 25 mg/mL DMSO stock solution and add to 900 μL corn oil, mix well to obtain a clear or suspension solution (2.5 mg/mL, ready for use in animals). Injection Formulation 4: DMSO : 20% SBE-β-CD in saline = 10 : 90 [i.e. 100 μL DMSO → 900 μL (20% SBE-β-CD in saline)] *Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Injection Formulation 5: 2-Hydroxypropyl-β-cyclodextrin : Saline = 50 : 50 (i.e. 500 μL 2-Hydroxypropyl-β-cyclodextrin → 500 μL Saline) Injection Formulation 6: DMSO : PEG300 : castor oil : Saline = 5 : 10 : 20 : 65 (i.e. 50 μL DMSO → 100 μLPEG300 → 200 μL castor oil → 650 μL Saline) Injection Formulation 7: Ethanol : Cremophor : Saline = 10: 10 : 80 (i.e. 100 μL Ethanol → 100 μL Cremophor → 800 μL Saline) Injection Formulation 8: Dissolve in Cremophor/Ethanol (50 : 50), then diluted by Saline Injection Formulation 9: EtOH : Corn oil = 10 : 90 (i.e. 100 μL EtOH → 900 μL Corn oil) Injection Formulation 10: EtOH : PEG300:Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL EtOH → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Oral Formulations Oral Formulation 1: Suspend in 0.5% CMC Na (carboxymethylcellulose sodium) Oral Formulation 2: Suspend in 0.5% Carboxymethyl cellulose Example: Take the Oral Formulation 1 (Suspend in 0.5% CMC Na) as an example, if 100 mL of 2.5 mg/mL working solution is to be prepared, you can first prepare 0.5% CMC Na solution by measuring 0.5 g CMC Na and dissolve it in 100 mL ddH2O to obtain a clear solution; then add 250 mg of the product to 100 mL 0.5% CMC Na solution, to make the suspension solution (2.5 mg/mL, ready for use in animals). Oral Formulation 3: Dissolved in PEG400 Oral Formulation 4: Suspend in 0.2% Carboxymethyl cellulose Oral Formulation 5: Dissolve in 0.25% Tween 80 and 0.5% Carboxymethyl cellulose Oral Formulation 6: Mixing with food powders Note: Please be aware that the above formulations are for reference only. InvivoChem strongly recommends customers to read literature methods/protocols carefully before determining which formulation you should use for in vivo studies, as different compounds have different solubility properties and have to be formulated differently. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 0.5909 mL | 2.9545 mL | 5.9091 mL | |
| 5 mM | 0.1182 mL | 0.5909 mL | 1.1818 mL | |
| 10 mM | 0.0591 mL | 0.2955 mL | 0.5909 mL |