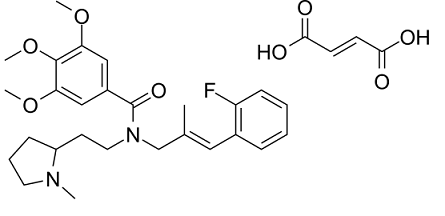
VUF11207 fumarate is a novel, selective and highly potent agonist of CXCR7 (pKi of 8.1) , inducing the recruitment of β-arrestin2 (pEC50 of 8.8) and subsequent internalization (pEC50 of 7.9) of CXCR7.
Physicochemical Properties
| Molecular Formula | C31H39FN2O8 |
| Molecular Weight | 586.648372888565 |
| Exact Mass | 586.27 |
| CAS # | 1785665-61-3 |
| Related CAS # | 1785665-61-3 |
| PubChem CID | 90488970 |
| Appearance | Light yellow to yellow solid powder |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 10 |
| Rotatable Bond Count | 12 |
| Heavy Atom Count | 42 |
| Complexity | 780 |
| Defined Atom Stereocenter Count | 0 |
| SMILES | C/C(=C\C1=CC=CC=C1F)/CN(CCC2CCCN2C)C(=O)C3=CC(=C(C(=C3)OC)OC)OC.C(=C/C(=O)O)\C(=O)O |
| InChi Key | MTPOECCHJLVVGO-FDULSSLPSA-N |
| InChi Code | InChI=1S/C27H35FN2O4.C4H4O4/c1-19(15-20-9-6-7-11-23(20)28)18-30(14-12-22-10-8-13-29(22)2)27(31)21-16-24(32-3)26(34-5)25(17-21)33-4;5-3(6)1-2-4(7)8/h6-7,9,11,15-17,22H,8,10,12-14,18H2,1-5H3;1-2H,(H,5,6)(H,7,8)/b19-15+;2-1+ |
| Chemical Name | (E)-but-2-enedioic acid;N-[(E)-3-(2-fluorophenyl)-2-methylprop-2-enyl]-3,4,5-trimethoxy-N-[2-(1-methylpyrrolidin-2-yl)ethyl]benzamide |
| Synonyms | VUF-11207 fumarate; VUF11207 fumarate; VUF 11207 fumarate |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month Note: Please store this product in a sealed and protected environment, avoid exposure to moisture. |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | CXCR7 ( pKi = 8.1 ) |
| ln Vitro | VUF11207 fumarate Fumarate (0.17 nM; 5 days) blocks RANKL- and TNF-α-induced osteoclastogenesis by blocking CXCL12 in osteoclast precursor cells [1]. Cell Viability Assay [1] Cell Line: Osteoclast Precursor Cells (RANKL- and TnF-α-induced) Concentration: 0.17 nM (100 ng/mL) Incubation Time: 5 Phosphorylation of erk to inhibit osteoclastogenesis [1]. Day results: showed inhibitory effect on CXCL12. |
| ln Vivo | VUF11207 fumarate (100 μg/day; SC; once daily for 5 days) LPS-induced osteoclastogenesis, bone resorption, and RANKL and TNF-α production in cyclohexane [1]. : Male c57Bl/6J wild-type/WT mice (8-10 weeks old; 20-25 g; LPS induced) [1] Dose: 100 µg/day Administration: SC; once daily for 5 days Results: The number of osteoclasts was significantly reduced, and the expression levels of cathepsin K mRNA, ranKl and TnF-α mRNA were inhibited. Reduce LPS-induced bone resorption area. |
| Cell Assay |
Cell Line: Osteoclast precursor cells (RANKL‑ and TnF‑α‑induced) Concentration: 0.17 nM (100 ng/mL) Incubation Time: 5 days Result: Showed inhibitory effect on CXCL12. |
| Animal Protocol |
Male c57Bl/6J wild‑type/WT mice (8‑10‑week‑old; 20‑25 g; LPS-induced) 100 µg/day Subcutaneous injection; single daily for 5 days |
| References |
[1]. Synthesis, modeling and functional activity of substituted styrene-amides as small-molecule CXCR7 agonists. Eur J Med Chem. 2012 May;51:184-92. [2]. C‑X‑C receptor 7 agonist acts as a C‑X‑C motif chemokine ligand 12 inhibitor to ameliorate osteoclastogenesis and bone resorption. Mol Med Rep. 2022 Mar;25(3):78. |
Solubility Data
| Solubility (In Vitro) | DMSO: ~100 mg/mL (~170.5 mM) |
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.08 mg/mL (3.55 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 20.8 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 2.08 mg/mL (3.55 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 20.8 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 3: ≥ 2.08 mg/mL (3.55 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 20.8 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.7046 mL | 8.5230 mL | 17.0459 mL | |
| 5 mM | 0.3409 mL | 1.7046 mL | 3.4092 mL | |
| 10 mM | 0.1705 mL | 0.8523 mL | 1.7046 mL |