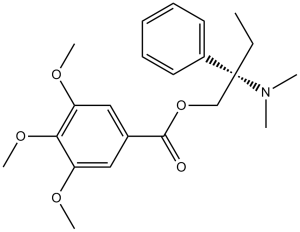
Trimebutine (Mebutin; Debridat; Polibutin; Modulon; Dromostat; Ibutin; Polibutin; TM 906; Trimedat) is a potent agonist of peripheral mu, kappa and delta (μ, κ, and δ) opioid receptors. It has been used to treat both acute and persistent abdominal pain as a spasmolytic agent. For the treatment of irritable bowel syndrome and other gastrointestinal disorders, trimebutine salt (maleic acid) is sold under the brands Debridat, Recutin, Polybutin, or Modulon. A premature activation of phase III of the migrating motor complex in the digestive tract is one way that trimebutine works.
Physicochemical Properties
| Molecular Formula | C22H29NO5 | |
| Molecular Weight | 387.47 | |
| Exact Mass | 387.204 | |
| CAS # | 39133-31-8 | |
| Related CAS # | Trimebutine maleate; 34140-59-5; Trimebutine-d5 fumarate; 2747915-18-8 | |
| PubChem CID | 5573 | |
| Appearance | White to off-white solid powder | |
| Density | 1.1±0.1 g/cm3 | |
| Boiling Point | 457.9±34.0 °C at 760 mmHg | |
| Melting Point | 79ºC | |
| Flash Point | 230.8±25.7 °C | |
| Vapour Pressure | 0.0±1.1 mmHg at 25°C | |
| Index of Refraction | 1.534 | |
| LogP | 4.34 | |
| Hydrogen Bond Donor Count | 0 | |
| Hydrogen Bond Acceptor Count | 6 | |
| Rotatable Bond Count | 10 | |
| Heavy Atom Count | 28 | |
| Complexity | 466 | |
| Defined Atom Stereocenter Count | 0 | |
| InChi Key | LORDFXWUHHSAQU-UHFFFAOYSA-N | |
| InChi Code | InChI=1S/C22H29NO5/c1-7-22(23(2)3,17-11-9-8-10-12-17)15-28-21(24)16-13-18(25-4)20(27-6)19(14-16)26-5/h8-14H,7,15H2,1-6H3 | |
| Chemical Name | [2-(dimethylamino)-2-phenylbutyl] 3,4,5-trimethoxybenzoate | |
| Synonyms |
|
|
| HS Tariff Code | 2934.99.9001 | |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | μ-opiate receptor; kappa-opiate receptor; delta-opiate receptor |
| ADME/Pharmacokinetics |
Absorption, Distribution and Excretion The free base form or salt form of trimebutine are rapidly absorbed after oral administration, with the peak plasma concentration reached after 1 hour of ingestion. The time to reach peak plasma concentration following a single oral dose of 200mg trimebutine is 0.80 hours. Renal elimination is predominant while excretion into feces is also observed (5-12%). About 94% of an oral dose of trimebutine is eliminated by the kidneys in the form of various metabolites and less than 2.4% of total ingested drug is recovered as unchanged parent drug in the urine. Trimebutine is most likely to be accumulated in the stomach and the intestinal walls in highest concentrations. The fetal transfer is reported to be low. Metabolism / Metabolites Trimebutine undergoes extensive hepatic first-pass metabolism. Nortrimebutine, or N-monodesmethyltrimebutine, is the main metabolite that retains pharmacological activity on the colon. This metabolite can undergo second N-demethylation to form N-didesmethyltrimebutine. Other main urinary metabolites (2-amino, 2-methylamino or 2-dimethylamino-2-phenylbutan-1-ol) can be formed via hydrolysis of the ester bond of desmethylated metabolites or initial hydrolysis of the ester bond of trimebutine followed by sequential N-demethylation. Trimebutine is also prone to sulphate and/or glucuronic acid conjugation. Biological Half-Life The elimination half life is approximately 1 hour following a single oral dose of 2mg/kg, and 2.77 hours following a single oral dose 200 mg. |
| Toxicity/Toxicokinetics |
Protein Binding Protein binding is minimal with 5% in vivo and in vitro to serum albumin. |
| References |
[1]. The opioid receptor selectivity for trimebutine in isolated tissues experiments and receptor binding studies. J Pharmacobiodyn, 1990. 13(7): p. 448-53. [2]. Pharmacological properties of trimebutine and N-monodesmethyltrimebutine. J Pharmacol Exp Ther, 1999. 289(3): p. 1391-7. [3]. Effectiveness of prokinetic agents against diseases external to the gastrointestinal tract. J Gastroenterol Hepatol, 2009. 24(4): p. 537-46. |
| Additional Infomation |
3,4,5-trimethoxybenzoic acid [2-(dimethylamino)-2-phenylbutyl] ester is a trihydroxybenzoic acid. Trimebutine is a spasmolytic agent that regulates intestinal and colonic motility and relieves abdominal pain with antimuscarinic and weak mu opioid agonist effects. It is marketed for the treatment of irritable bowel syndrome (IBS) and lower gastrointestinal tract motility disorders, with IBS being one of the most common multifactorial GI disorders. It is used to restore normal bowel function and is commonly present in pharmaceutical mixtures as trimebutine maleate salt form. Trimebutine is not a FDA-approved drug, but it is available in Canada and several other international countries. Proposed spasmolytic with possible local anesthetic action used in gastrointestinal disorders. Drug Indication Indicated for symptomatic treatment of irritable bowel syndrome (IBS) and treatment of postoperative paralytic ileus following abdominal surgery. Mechanism of Action At high concentrations, trimebutine is shown to inhibit the extracellular Ca2+ influx in the smooth muscle cells through voltage dependent L-type Ca2+ channels and further Ca2+ release from intracellular Ca2+ stores. Trimebutine is suggested to bind to the inactivated state of the calcium channel with high affinity. Reduced calcium influx attenuates membrane depolarization and decrease colon peristalsis. It also inhibits outward K+ currents in response to membrane depolarization of the GI smooth muscle cells at resting conditions through inhibition of delayed rectifier K+ channels and Ca2+ dependent K+ channels, which results in induced muscle contractions. Trimebutine binds to mu opioid receptors with more selectivity compared to delta or kappa opioid receptors but with lower affinity than their natural ligands. Its metabolites (N-monodesmethyl-trimebutine or nor-trimebutine), are also shown to bind to opoid receptors on brain membranes and myenteric synaptosomes. |
Solubility Data
| Solubility (In Vitro) |
|
|||
| Solubility (In Vivo) |
Note: Listed below are some common formulations that may be used to formulate products with low water solubility (e.g. < 1 mg/mL), you may test these formulations using a minute amount of products to avoid loss of samples. Injection Formulations (e.g. IP/IV/IM/SC) Injection Formulation 1: DMSO : Tween 80: Saline = 10 : 5 : 85 (i.e. 100 μL DMSO stock solution → 50 μL Tween 80 → 850 μL Saline) *Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH ₂ O to obtain a clear solution. Injection Formulation 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL DMSO → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Injection Formulation 3: DMSO : Corn oil = 10 : 90 (i.e. 100 μL DMSO → 900 μL Corn oil) Example: Take the Injection Formulation 3 (DMSO : Corn oil = 10 : 90) as an example, if 1 mL of 2.5 mg/mL working solution is to be prepared, you can take 100 μL 25 mg/mL DMSO stock solution and add to 900 μL corn oil, mix well to obtain a clear or suspension solution (2.5 mg/mL, ready for use in animals). Injection Formulation 4: DMSO : 20% SBE-β-CD in saline = 10 : 90 [i.e. 100 μL DMSO → 900 μL (20% SBE-β-CD in saline)] *Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Injection Formulation 5: 2-Hydroxypropyl-β-cyclodextrin : Saline = 50 : 50 (i.e. 500 μL 2-Hydroxypropyl-β-cyclodextrin → 500 μL Saline) Injection Formulation 6: DMSO : PEG300 : castor oil : Saline = 5 : 10 : 20 : 65 (i.e. 50 μL DMSO → 100 μLPEG300 → 200 μL castor oil → 650 μL Saline) Injection Formulation 7: Ethanol : Cremophor : Saline = 10: 10 : 80 (i.e. 100 μL Ethanol → 100 μL Cremophor → 800 μL Saline) Injection Formulation 8: Dissolve in Cremophor/Ethanol (50 : 50), then diluted by Saline Injection Formulation 9: EtOH : Corn oil = 10 : 90 (i.e. 100 μL EtOH → 900 μL Corn oil) Injection Formulation 10: EtOH : PEG300:Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL EtOH → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Oral Formulations Oral Formulation 1: Suspend in 0.5% CMC Na (carboxymethylcellulose sodium) Oral Formulation 2: Suspend in 0.5% Carboxymethyl cellulose Example: Take the Oral Formulation 1 (Suspend in 0.5% CMC Na) as an example, if 100 mL of 2.5 mg/mL working solution is to be prepared, you can first prepare 0.5% CMC Na solution by measuring 0.5 g CMC Na and dissolve it in 100 mL ddH2O to obtain a clear solution; then add 250 mg of the product to 100 mL 0.5% CMC Na solution, to make the suspension solution (2.5 mg/mL, ready for use in animals). Oral Formulation 3: Dissolved in PEG400 Oral Formulation 4: Suspend in 0.2% Carboxymethyl cellulose Oral Formulation 5: Dissolve in 0.25% Tween 80 and 0.5% Carboxymethyl cellulose Oral Formulation 6: Mixing with food powders Note: Please be aware that the above formulations are for reference only. InvivoChem strongly recommends customers to read literature methods/protocols carefully before determining which formulation you should use for in vivo studies, as different compounds have different solubility properties and have to be formulated differently. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.5808 mL | 12.9042 mL | 25.8084 mL | |
| 5 mM | 0.5162 mL | 2.5808 mL | 5.1617 mL | |
| 10 mM | 0.2581 mL | 1.2904 mL | 2.5808 mL |