Terbutaline Sulfate (Terbutaline hemisulfate; Bronclyn, Brethine, KWD-2019; KWD2019; Bricanyl, Brethaire, Terbulin) is a potent and selective β2-adrenergic receptor agonist used for the treatment of allergic asthma. IIt has an IC50 of 53 NM for β2-adrenergic receptor activation, while it has negligible or no effect on alpha-adrenergic receptors. The medication preferentially affects β2-adrenergic receptors, but it stimulates beta-adrenergic receptors less selectively than beta2-agonists, which are more selectively stimulating. The clinical efficacy of terbutaline in the treatment of allergic asthma is attributed to its inhibition of antigen-induced histamine release from human lung tissue that has been passively sensitized.
Physicochemical Properties
| Molecular Formula | C12H20NO5S0.5 | |
| Molecular Weight | 548.65 | |
| Exact Mass | 548.24 | |
| Elemental Analysis | C, 52.54; H, 7.35; N, 5.11; O, 29.16; S, 5.84 | |
| CAS # | 23031-32-5 | |
| Related CAS # | Terbutaline; 23031-25-6 | |
| PubChem CID | 441334 | |
| Appearance | White to off-white crystalline powder | |
| Density | 1.1840 (rough estimate) | |
| Boiling Point | 419.2ºC at 760 mmHg | |
| Melting Point | 246-248ºC | |
| Flash Point | 165.3ºC | |
| Vapour Pressure | 8.92E-08mmHg at 25°C | |
| Index of Refraction | 1.6900 (estimate) | |
| LogP | 4.248 | |
| Hydrogen Bond Donor Count | 10 | |
| Hydrogen Bond Acceptor Count | 12 | |
| Rotatable Bond Count | 8 | |
| Heavy Atom Count | 37 | |
| Complexity | 286 | |
| Defined Atom Stereocenter Count | 0 | |
| SMILES | S(=O)(=O)(O[H])O[H].O([H])C([H])(C1C([H])=C(C([H])=C(C=1[H])O[H])O[H])C([H])([H])N([H])C(C([H])([H])[H])(C([H])([H])[H])C([H])([H])[H].O([H])C([H])(C1C([H])=C(C([H])=C(C=1[H])O[H])O[H])C([H])([H])N([H])C(C([H])([H])[H])(C([H])([H])[H])C([H])([H])[H] |
|
| InChi Key | KFVSLSTULZVNPG-UHFFFAOYSA-N | |
| InChi Code | InChI=1S/2C12H19NO3.H2O4S/c2*1-12(2,3)13-7-11(16)8-4-9(14)6-10(15)5-8;1-5(2,3)4/h2*4-6,11,13-16H,7H2,1-3H3;(H2,1,2,3,4) | |
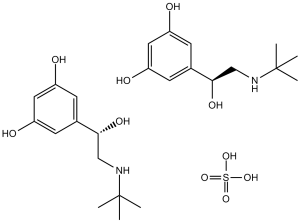
| Chemical Name | 5-(2-(tert-butylamino)-1-hydroxyethyl)benzene-1,3-diol; sulfate (2:1) | |
| Synonyms |
|
|
| HS Tariff Code | 2934.99.9001 | |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month Note: Please store this product in a sealed and protected environment, avoid exposure to moisture. |
|
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | β adrenergic receptor ( IC50 = 53 nM ) | |
| ln Vitro |
|
|
| ln Vivo |
|
|
| Cell Assay |
Cell Line: J774 macrophages Concentration: 0-10 μM Incubation Time: 1 hour Result: Enhanced MKP-1 expression in J774 macrophages in a dose-dependent manner. |
|
| Animal Protocol |
Adult male ob/ob mice 0.5 mg/kg Intraperitoneal injection; 0.5 mg/kg; twice a day; 20 days |
|
| Toxicity/Toxicokinetics |
Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation Maternal use of oral or inhaled terbutaline is unlikely to affect a breastfed infant. The authors of several reviews and expert guidelines agree that use of inhaled bronchodilators is acceptable during breastfeeding because of the low bioavailability and maternal serum levels after use. Terbutaline use as a tocolytic agent might decrease the duration of breastfeeding. ◉ Effects in Breastfed Infants Two papers have reported a total of 4 infants aged 3 to 8 weeks who were breastfed during maternal use of oral terbutaline 2.5 or 5 mg three times daily. None of the infants had any signs of sympathetic stimulation and all were developing normally. These cases were also summarized in a third publication. ◉ Effects on Lactation and Breastmilk A small retrospective survey from Serbia found that mothers who received a beta agonist pharmacologically similar to terbutaline (fenoterol or hexoprenaline) as a tocolytic breastfed for a shorter period of time than those who received no tocolytic (4.5 vs 9.5 months). It is not known if terbutaline has a similar effect. A study in an Australian hospital compared breastfeeding outcomes in women who received a cesarean section during 2 time periods. During the first time period women did not receive terbutaline before a category one or two cesarean section (n = 423). In the second period, all women receiving a category one or two cesarean section received terbutaline 250 mcg subcutaneously as a tocolytic agent unless there was a contraindication at the time a decision was made to perform a cesarean section (n = 253). The breastfeeding rates at the time of discharge were 95% in the first period and 99% in the second period. The difference was statistically significant. |
|
| References |
[1]. Allergy . 1984 Jul;39(5):351-7. [2]. J Enzyme Inhib Med Chem . 2004 Apr;19(2):113-7. [3]. Signaling Through Hepatocyte Vasopressin Receptor 1 Protects Mouse Liver From Ischemia-Reperfusion Injury. Oncotarget. 2016 Oct 25;7(43):69276-69290. [4]. Int J Clin Pharmacol Ther Toxicol . 1992 Sep;30(9):342-62. |
|
| Additional Infomation |
Terbutaline sulfate is an ethanolamine sulfate salt. It is functionally related to a terbutaline. Terbutaline Sulfate is the sulfate salt form of terbutaline, an ethanolamine derivative with bronchodilating and tocolytic properties. Terbutaline sulfate selectively binds to and activates beta-2 adrenergic receptors, leading to intracellular adenyl cyclase activation via a trimeric G protein and subsequent increase in cyclic cAMP production. Increased cAMP levels result in relaxation of bronchial and vascular smooth muscle mediated through the activation of protein kinase A (PKA), which phosphorylates proteins in control of muscle tone. cAMP also inhibits calcium ion release from intracellular stores, reduces calcium entry into cells and induces the sequestration of intracellular calcium all of which aids the relaxation of airway muscles. Terbutaline sulfate also increases mucociliary clearance and reduces release of inflammatory cell mediators. A selective beta-2 adrenergic agonist used as a bronchodilator and tocolytic. See also: Terbutaline (has active moiety). |
Solubility Data
| Solubility (In Vitro) |
|
|||
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 0.5 mg/mL (1.82 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 5.0 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 0.5 mg/mL (1.82 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 5.0 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 3: ≥ 0.5 mg/mL (1.82 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 5.0 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. Solubility in Formulation 4: 120 mg/mL (437.45 mM) in PBS (add these co-solvents sequentially from left to right, and one by one), clear solution; with ultrasonication. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.8227 mL | 9.1133 mL | 18.2266 mL | |
| 5 mM | 0.3645 mL | 1.8227 mL | 3.6453 mL | |
| 10 mM | 0.1823 mL | 0.9113 mL | 1.8227 mL |