SR-4835 (SR4835) is a novel highly selective dual inhibitor of CDK12 and CDK13 with anticancer activity. SR-4835 induces triple-negative breast cancer (TNBC) cell death by working in concert with PARP inhibitors and chemotherapy that damages DNA. The suppression of core DNA damage response proteins is caused by intronic polyadenylation site cleavage, which is triggered by the inhibition or loss of CDK12/CDK13. This induces a phenotype known as "BRCAness," which leads to deficiencies in the repair of DNA damage and works in concert with PARP inhibitors and chemotherapy that damages DNA.
Physicochemical Properties
| Molecular Formula | C21H20CL2N10O |
| Molecular Weight | 499.3559 |
| Exact Mass | 498.12 |
| Elemental Analysis | C, 50.51; H, 4.04; Cl, 14.20; N, 28.05; O, 3.20 |
| CAS # | 2387704-62-1 |
| Related CAS # | 2387704-62-1 |
| PubChem CID | 139600338 |
| Appearance | Off-white to light brown solid powder |
| LogP | 2.8 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 5 |
| Heavy Atom Count | 34 |
| Complexity | 705 |
| Defined Atom Stereocenter Count | 0 |
| InChi Key | FSELUFUYNUNZKD-UHFFFAOYSA-N |
| InChi Code | InChI=1S/C21H20Cl2N10O/c1-31-10-12(8-26-31)33-11-25-18-19(29-21(30-20(18)33)32-2-4-34-5-3-32)24-9-17-27-15-6-13(22)14(23)7-16(15)28-17/h6-8,10-11H,2-5,9H2,1H3,(H,27,28)(H,24,29,30) |
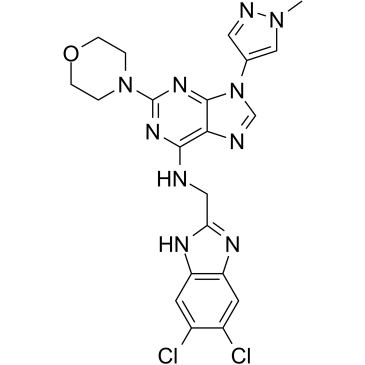
| Chemical Name | N-[(5,6-dichloro-1H-benzimidazol-2-yl)methyl]-9-(1-methylpyrazol-4-yl)-2-morpholin-4-ylpurin-6-amine |
| Synonyms | SR4835; SR 4835; SR-4835 |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | CDK12 (IC50 = 99 nM); CDK12 (Kd = 99 nM); CDK13 (Kd = 4.9 nM) |
| ln Vitro | SR-4835 is a powerful and specific dual inhibitor of CDK12 and CDK13 that inhibits DDR protein expression. To cause TNBC cell death, CDK12/CDK13 inhibition works in concert with PARP inhibition or DNA-damaging agents.[1] |
| ln Vivo | SR-4835 has potent anti-TNBC activity in vivo and increases the anti-tumor activity of olaparib, the treatment of choice for TNBC. After prolonged dosage, SR-4835 is well tolerated in mice. SR-4835 collaborates with chemotherapy drugs that break DNA.[1] |
| Cell Assay | The Clonogenic Assays. Three duplicates of six-well plates with 500 cells per well are plated. The cells are incubated for seven to ten days, with a change of medium every two to three days. Following an overnight incubation period, SR-4835 is added to the medium and incubated for an additional 72 hours. |
| Animal Protocol |
Female SCID Beige mice bearing xenograft tumor fragments, male C57Bl-6 mice 20 mg/kg Oral gavage, IV |
| References |
[1]. Therapeutic Targeting of CDK12/CDK13 in Triple-Negative Breast Cancer. Cancer Cell. 2019 Oct 8. pii: S1535-6108(19)30424-6. |
Solubility Data
| Solubility (In Vitro) |
DMSO: 23~100 mg/mL (46.06~200.3 mM) H2O: ~1 mg/mL (~2.00 mM) |
| Solubility (In Vivo) |
Solubility in Formulation 1: 2 mg/mL (4.01 mM) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), suspension solution; with sonication. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 20.0 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 0.89 mg/mL (1.78 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 8.9 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. Solubility in Formulation 3: 5 mg/mL (10.01 mM) in 20% HP-β-CD in Saline (add these co-solvents sequentially from left to right, and one by one), suspension solution; with ultrasonication. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.0026 mL | 10.0128 mL | 20.0256 mL | |
| 5 mM | 0.4005 mL | 2.0026 mL | 4.0051 mL | |
| 10 mM | 0.2003 mL | 1.0013 mL | 2.0026 mL |