SCH-563705 (SCH563705) is a novel potent and orally bioactive antagonist of CXCR2 and CXCR1 with anti-Inflammatory and immunomodulatory activity. It has favorable oral pharmacokinetic profiles in dogs, rats, mice, and monkeys. With regard to CXCR2 and CXCR1, SCH-563705 exhibits IC50 values of 1.3 nM, 7.3 nM, and Ki values of 1 and 3 nM, respectively. The application of the CXCR2/CXCR1 antagonist SCH563705 to target neutrophil migration resulted in a dose-dependent reduction in clinical disease scores and measurements of paw thickness. Additionally, histopathology and paw cytokine analyses demonstrated a clear reduction in inflammation and the degradation of bone and cartilage. On the other hand, the CCR2 antagonist MK0812, which targets monocyte migration, had no effect on the severity of arthritis disease. The effects of both SCH563705 and MK0812 on the peripheral blood neutrophil and monocyte populations were used to confirm their pharmacodynamic activities. SCH563705 increased the CXCL1 ligand and selectively decreased the frequency of neutrophils in peripheral blood. MK0812 caused an increase in the CCR2 ligand CCL2 and a selective decrease in the frequency of peripheral blood monocytes. The therapeutic potential for targeting CXCR2/CXCR1 in human arthritides is highlighted by the significantly greater impact of CXCR2/CXCR1 antagonism in this model of arthritis compared to CCR2 antagonism.
Physicochemical Properties
| Molecular Formula | C23H27N3O5 | |
| Molecular Weight | 425.49 | |
| Exact Mass | 425.195 | |
| Elemental Analysis | C, 64.93; H, 6.40; N, 9.88; O, 18.80 | |
| CAS # | 473728-58-4 | |
| Related CAS # |
|
|
| PubChem CID | 10310100 | |
| Appearance | White to off-white solid powder | |
| Density | 1.29g/cm3 | |
| Boiling Point | 555.9ºC at 760 mmHg | |
| Flash Point | 290ºC | |
| Index of Refraction | 1.615 | |
| LogP | 3.79 | |
| Hydrogen Bond Donor Count | 3 | |
| Hydrogen Bond Acceptor Count | 7 | |
| Rotatable Bond Count | 8 | |
| Heavy Atom Count | 31 | |
| Complexity | 748 | |
| Defined Atom Stereocenter Count | 1 | |
| SMILES | O=C(C(NC1=CC=CC(C(N(C)C)=O)=C1O)=C2N[C@H](CC)C3=CC(C(C)C)=CO3)C2=O |
|
| InChi Key | DGKQQEVYYPCMNE-OAHLLOKOSA-N | |
| InChi Code | InChI=1S/C23H27N3O5/c1-6-15(17-10-13(11-31-17)12(2)3)24-18-19(22(29)21(18)28)25-16-9-7-8-14(20(16)27)23(30)26(4)5/h7-12,15,24-25,27H,6H2,1-5H3/t15-/m1/s1 | |
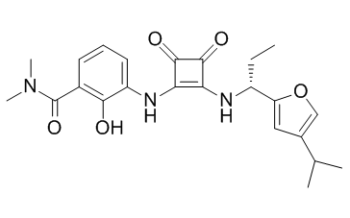
| Chemical Name | 3-[[3,4-dioxo-2-[[(1R)-1-(4-propan-2-ylfuran-2-yl)propyl]amino]cyclobuten-1-yl]amino]-2-hydroxy-N,N-dimethylbenzamide | |
| Synonyms |
|
|
| HS Tariff Code | 2934.99.9001 | |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | CXCR2 ( Ki = 1 nM ); CXCR1 ( Ki = 3 nM ); CXCR2 ( Ki = 1.3 nM ); CXCR1 ( Ki = 7.3 nM ); Mouse CXCR2 ( Ki = 5.2 nM ) | |
| ln Vitro |
|
|
| ln Vivo |
|
|
| Enzyme Assay | A novel series of cyclobutenedione centered C(4)-alkyl substituted furanyl analogs was developed as potent CXCR2 and CXCR1 antagonists. Compound 16 exhibits potent inhibitory activities against IL-8 binding to the receptors (CXCR2 Ki=1 nM, IC(50)=1.3 nM; CXCR1 Ki=3 nM, IC(50)=7.3 nM), and demonstrates potent inhibition against both Gro-alpha and IL-8 induced hPMN migration (chemotaxis: CXCR2 IC(50)=0.5 nM, CXCR1 IC(50)=37 nM). In addition, 16 has shown good oral pharmacokinetic profiles in rat, mouse, monkey, and dog.[1] | |
| Cell Assay | SCH563705 selectively reduced the peripheral blood neutrophil frequency, and caused an elevation in the CXCR2 ligand CXCL1. MK0812 selectively reduced the peripheral blood monocyte frequency, and caused an elevation in the CCR2 ligand CCL2. The much greater impact of CXCR2/CXCR1 antagonism relative to CCR2 antagonism in this model of arthritis highlights the therapeutic potential for targeting CXCR2/CXCR1 in human arthritides.[2] | |
| Animal Protocol |
|
|
| References |
[1]. C(4)-alkyl substituted furanyl cyclobutenediones as potent, orally bioavailable CXCR2 and CXCR1 receptor antagonists. Bioorg Med Chem Lett. 2007 Jul 1;17(13):3778-83. [2]. Pharmacological targeting reveals distinct roles for CXCR2/CXCR1 and CCR2 in a mouse model of arthritis. Biochem Biophys Res Commun. 2010 Jan 1;391(1):1080-6. |
|
| Additional Infomation | Neutrophils and monocytes are abundantly represented in the synovial fluid and tissue in rheumatoid arthritis patients. We therefore explored the effects of small molecule chemokine receptor antagonists to block migration of these cells in anti-collagen antibody-induced arthritis. Targeting neutrophil migration with the CXCR2/CXCR1 antagonist SCH563705 led to a dose-dependent decrease in clinical disease scores and paw thickness measurements and clearly reduced inflammation and bone and cartilage degradation based on histopathology and paw cytokine analyses. In contrast, targeting monocyte migration with the CCR2 antagonist MK0812 had no effect on arthritis disease severity. The pharmacodynamic activities of both SCH563705 and MK0812 were verified by assessing their effects on the peripheral blood monocyte and neutrophil populations. SCH563705 selectively reduced the peripheral blood neutrophil frequency, and caused an elevation in the CXCR2 ligand CXCL1. MK0812 selectively reduced the peripheral blood monocyte frequency, and caused an elevation in the CCR2 ligand CCL2. The much greater impact of CXCR2/CXCR1 antagonism relative to CCR2 antagonism in this model of arthritis highlights the therapeutic potential for targeting CXCR2/CXCR1 in human arthritides.[2] |
Solubility Data
| Solubility (In Vitro) |
|
|||
| Solubility (In Vivo) |
Note: Listed below are some common formulations that may be used to formulate products with low water solubility (e.g. < 1 mg/mL), you may test these formulations using a minute amount of products to avoid loss of samples. Injection Formulations (e.g. IP/IV/IM/SC) Injection Formulation 1: DMSO : Tween 80: Saline = 10 : 5 : 85 (i.e. 100 μL DMSO stock solution → 50 μL Tween 80 → 850 μL Saline) *Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH ₂ O to obtain a clear solution. Injection Formulation 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL DMSO → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Injection Formulation 3: DMSO : Corn oil = 10 : 90 (i.e. 100 μL DMSO → 900 μL Corn oil) Example: Take the Injection Formulation 3 (DMSO : Corn oil = 10 : 90) as an example, if 1 mL of 2.5 mg/mL working solution is to be prepared, you can take 100 μL 25 mg/mL DMSO stock solution and add to 900 μL corn oil, mix well to obtain a clear or suspension solution (2.5 mg/mL, ready for use in animals). Injection Formulation 4: DMSO : 20% SBE-β-CD in saline = 10 : 90 [i.e. 100 μL DMSO → 900 μL (20% SBE-β-CD in saline)] *Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Injection Formulation 5: 2-Hydroxypropyl-β-cyclodextrin : Saline = 50 : 50 (i.e. 500 μL 2-Hydroxypropyl-β-cyclodextrin → 500 μL Saline) Injection Formulation 6: DMSO : PEG300 : castor oil : Saline = 5 : 10 : 20 : 65 (i.e. 50 μL DMSO → 100 μLPEG300 → 200 μL castor oil → 650 μL Saline) Injection Formulation 7: Ethanol : Cremophor : Saline = 10: 10 : 80 (i.e. 100 μL Ethanol → 100 μL Cremophor → 800 μL Saline) Injection Formulation 8: Dissolve in Cremophor/Ethanol (50 : 50), then diluted by Saline Injection Formulation 9: EtOH : Corn oil = 10 : 90 (i.e. 100 μL EtOH → 900 μL Corn oil) Injection Formulation 10: EtOH : PEG300:Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL EtOH → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Oral Formulations Oral Formulation 1: Suspend in 0.5% CMC Na (carboxymethylcellulose sodium) Oral Formulation 2: Suspend in 0.5% Carboxymethyl cellulose Example: Take the Oral Formulation 1 (Suspend in 0.5% CMC Na) as an example, if 100 mL of 2.5 mg/mL working solution is to be prepared, you can first prepare 0.5% CMC Na solution by measuring 0.5 g CMC Na and dissolve it in 100 mL ddH2O to obtain a clear solution; then add 250 mg of the product to 100 mL 0.5% CMC Na solution, to make the suspension solution (2.5 mg/mL, ready for use in animals). Oral Formulation 3: Dissolved in PEG400 Oral Formulation 4: Suspend in 0.2% Carboxymethyl cellulose Oral Formulation 5: Dissolve in 0.25% Tween 80 and 0.5% Carboxymethyl cellulose Oral Formulation 6: Mixing with food powders Note: Please be aware that the above formulations are for reference only. InvivoChem strongly recommends customers to read literature methods/protocols carefully before determining which formulation you should use for in vivo studies, as different compounds have different solubility properties and have to be formulated differently. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.3502 mL | 11.7512 mL | 23.5023 mL | |
| 5 mM | 0.4700 mL | 2.3502 mL | 4.7005 mL | |
| 10 mM | 0.2350 mL | 1.1751 mL | 2.3502 mL |