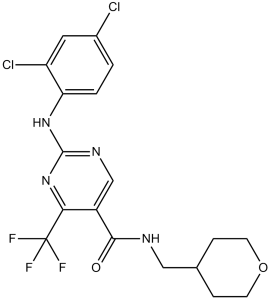
GW842166X (GW-842166X; GW842166; GW 842166X) is a novel, potent and highly selective agonist of cannabinoid receptor CB2 receptor with potential anti-inflammatory activity. It has the ability to treat inflammatory pain and activates CB2 at an EC50 of 63 nM.
Physicochemical Properties
| Molecular Formula | C18H17CL2F3N4O2 | |
| Molecular Weight | 449.25 | |
| Exact Mass | 448.068 | |
| Elemental Analysis | C, 48.12; H, 3.81; Cl, 15.78; F, 12.69; N, 12.47; O, 7.12 | |
| CAS # | 666260-75-9 | |
| Related CAS # |
|
|
| PubChem CID | 10253143 | |
| Appearance | White to off-white solid powder | |
| Density | 1.4±0.1 g/cm3 | |
| Index of Refraction | 1.571 | |
| LogP | 3.48 | |
| Hydrogen Bond Donor Count | 2 | |
| Hydrogen Bond Acceptor Count | 8 | |
| Rotatable Bond Count | 5 | |
| Heavy Atom Count | 29 | |
| Complexity | 552 | |
| Defined Atom Stereocenter Count | 0 | |
| SMILES | ClC1=CC(Cl)=C(NC2=NC=C(C(NCC3CCOCC3)=O)C(C(F)(F)F)=N2)C=C1 |
|
| InChi Key | TWQYWUXBZHPIIV-UHFFFAOYSA-N | |
| InChi Code | InChI=1S/C18H17Cl2F3N4O2/c19-11-1-2-14(13(20)7-11)26-17-25-9-12(15(27-17)18(21,22)23)16(28)24-8-10-3-5-29-6-4-10/h1-2,7,9-10H,3-6,8H2,(H,24,28)(H,25,26,27) | |
| Chemical Name | 2-(2,4-dichloroanilino)-N-(oxan-4-ylmethyl)-4-(trifluoromethyl)pyrimidine-5-carboxamide | |
| Synonyms | GW842166X; GW842166; GW 842166X; GW 842166; GW-842166X; 842166X; 842166; GW-842166 | |
| HS Tariff Code | 2934.99.9001 | |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | human CB2 ( IC50 = 63 nM ); rat CB2 ( IC50 = 91 nM ) | ||
| ln Vitro |
|
||
| ln Vivo |
|
||
| Animal Protocol |
|
||
| References |
[1]. Discovery of 2-[(2,4-dichlorophenyl)amino]-N-[(tetrahydro- 2H-pyran-4-yl)methyl]-4-(trifluoromethyl)- 5-pyrimidinecarboxamide, a selective CB2 receptor agonist for the treatment of inflammatory pain. J Med Chem. 2007 May 31;50(11):2597-6. |
||
| Additional Infomation |
5-pyrimidinecarboxamide, 2-[(2,4-dichlorophenyl)amino]-n-[(tetrahydro-2h-pyran-4-yl)methyl]-4-(trifluoromethyl)- is a dichlorobenzene. GW842166X has been used in trials studying the treatment of Pain, Analgesia, Inflammation, Osteoarthritis, and Pain, Inflammatory, among others. |
Solubility Data
| Solubility (In Vitro) |
|
|||
| Solubility (In Vivo) |
Solubility in Formulation 1: 2.5 mg/mL (5.56 mM) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), suspension solution; with sonication. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 2.5 mg/mL (5.56 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 3: ≥ 2.5 mg/mL (5.56 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. Solubility in Formulation 4: 30% Propylene glycol , 5% Tween 80 , 65% D5W: 30 mg/mL (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.2259 mL | 11.1297 mL | 22.2593 mL | |
| 5 mM | 0.4452 mL | 2.2259 mL | 4.4519 mL | |
| 10 mM | 0.2226 mL | 1.1130 mL | 2.2259 mL |