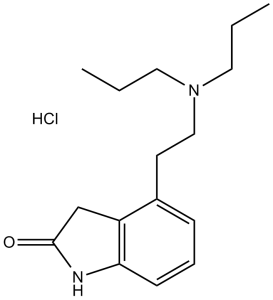
Ropinirole HCl (formerly SKF 101468; SKF-101468A; SKF 101468A; Requip), the hydrochloride salt of ropinirole, is a potent and selective dopamine D2 receptors agonist ( Ki = 29 nM) of the non-ergoline class of medications used for PD-Parkinson's disease, RLS/restless legs syndrome and extrapyramidal symptoms. It can also lessen the negative effects of selective serotonin reuptake inhibitors (SSRIs) and antipsychotics, such as Parkinsonism syndrome, erectile dysfunction, and sexual dysfunction. In the Fe2+-H2O2 reaction system, ropinirole scavenges free radicals and inhibits lipid peroxidation.
Physicochemical Properties
| Molecular Formula | C16H25CLN2O | |
| Molecular Weight | 296.84 | |
| Exact Mass | 296.165 | |
| Elemental Analysis | C, 64.74; H, 8.49; Cl, 11.94; N, 9.44; O, 5.39 | |
| CAS # | 91374-20-8 | |
| Related CAS # | Ropinirole; 91374-21-9; Ropinirole-d7 hydrochloride; 1261396-31-9; Ropinirole-d3 hydrochloride; 1329611-00-8 | |
| PubChem CID | 68727 | |
| Appearance | Light yellow solid powder | |
| Boiling Point | 410.5ºC at 760mmHg | |
| Melting Point | 241-243ºC | |
| LogP | 3.785 | |
| Hydrogen Bond Donor Count | 2 | |
| Hydrogen Bond Acceptor Count | 2 | |
| Rotatable Bond Count | 7 | |
| Heavy Atom Count | 20 | |
| Complexity | 287 | |
| Defined Atom Stereocenter Count | 0 | |
| SMILES | Cl.O=C1CC2C(=CC=CC=2CCN(CCC)CCC)N1 |
|
| InChi Key | XDXHAEQXIBQUEZ-UHFFFAOYSA-N | |
| InChi Code | InChI=1S/C16H24N2O.ClH/c1-3-9-18(10-4-2)11-8-13-6-5-7-15-14(13)12-16(19)17-15;/h5-7H,3-4,8-12H2,1-2H3,(H,17,19);1H | |
| Chemical Name | 4-[2-(dipropylamino)ethyl]-1,3-dihydroindol-2-one;hydrochloride | |
| Synonyms |
|
|
| HS Tariff Code | 2934.99.9001 | |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month Note: Please store this product in a sealed and protected environment, avoid exposure to moisture. |
|
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | D2 Receptor ( Ki = 0.7 nM ); hD2 Receptor ( pEC50 = 2.8 nM ); hD3 Receptor ( pEC50 = 2.8 nM ); hD4.4 Receptor ( pEC50 = 2.8 nM ) | |
| ln Vitro |
|
|
| ln Vivo |
|
|
| Animal Protocol |
Male Sprague–Dawley rats weighing 220-350 g 0.1, 1 or 10 mg/kg i.p. |
|
| References |
[1]. Pharmacol Biochem Behav . 1991 Jan;38(1):147-54. [2]. Brain Res . 1999 Aug 14;838(1-2):51-9. [3]. Brain Res . 2007 Jul 30:1160:113-23. [4]. Pharmacotherapy . 2000 Jan;20(1 Pt 2):17S-25S. [5]. Sleep . 2004 Aug 1;27(5):907-14. |
|
| Additional Infomation |
Ropinirole hydrochloride is a member of indoles. Ropinirole Hydrochloride is the hydrochloride salt form of ropinirole, a non-ergot dopamine agonist with antiparkinsonian property. Acting as a substitute for dopamine, ropinirole hydrochloride binds to and activates dopamine D2 and D3 receptors within the caudate putamen in the brain, thereby improving motor function. See also: Ropinirole (has active moiety). Drug Indication Meeting highlights from the Committee for Medicinal Products for Veterinary Use (CVMP) 5-7 October 202108/10/2021 |
Solubility Data
| Solubility (In Vitro) |
|
|||
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 1.67 mg/mL (5.63 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 16.7 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 1.67 mg/mL (5.63 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 16.7 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 3: ≥ 1.67 mg/mL (5.63 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 16.7 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. Solubility in Formulation 4: 100 mg/mL (336.88 mM) in PBS (add these co-solvents sequentially from left to right, and one by one), clear solution; with ultrasonication. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.3688 mL | 16.8441 mL | 33.6882 mL | |
| 5 mM | 0.6738 mL | 3.3688 mL | 6.7376 mL | |
| 10 mM | 0.3369 mL | 1.6844 mL | 3.3688 mL |