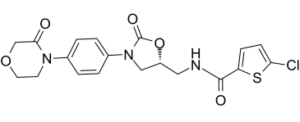
5-R-Rivaroxaban is (R)-enantiomer of Rivaroxaban (also known as BAY 59-7939) which is the first orally available, direct inhibitor of Factor Xa with Ki and IC50 of 0.4 nM and 0.7 nM in cell-free assays, respectively. Rivaroxaban binds to the Tyr288 in S1 pocket of factor Xa through the interaction of Tyr288 and the chlorine substituent of the chlorothiophene moiety. Rivaroxabanis used as an oral anticoagulant developed by Bayer amd was marketed in a number of countries with the brand name of Xarelto. Rivaroxaban is well absorbed from the gut and maximum inhibition of factor Xa occurs four hours after a dose. The effects lasts 8–12 hours, but factor Xa activity does not return to normal within 24 hours so once-daily dosing is possible.
Physicochemical Properties
| Molecular Formula | C19H18CLN3O5S | |
| Molecular Weight | 435.88 | |
| Exact Mass | 435.065 | |
| CAS # | 865479-71-6 | |
| Related CAS # |
|
|
| PubChem CID | 11524901 | |
| Appearance | Light yellow to yellow solid powder | |
| Density | 1.5±0.1 g/cm3 | |
| Boiling Point | 732.6±60.0 °C at 760 mmHg | |
| Flash Point | 396.9±32.9 °C | |
| Vapour Pressure | 0.0±2.4 mmHg at 25°C | |
| Index of Refraction | 1.633 | |
| LogP | 1.84 | |
| Hydrogen Bond Donor Count | 1 | |
| Hydrogen Bond Acceptor Count | 6 | |
| Rotatable Bond Count | 5 | |
| Heavy Atom Count | 29 | |
| Complexity | 645 | |
| Defined Atom Stereocenter Count | 1 | |
| SMILES | C1COCC(=O)N1C2=CC=C(C=C2)N3C[C@H](OC3=O)CNC(=O)C4=CC=C(S4)Cl |
|
| InChi Key | KGFYHTZWPPHNLQ-CQSZACIVSA-N | |
| InChi Code | InChI=1S/C19H18ClN3O5S/c20-16-6-5-15(29-16)18(25)21-9-14-10-23(19(26)28-14)13-3-1-12(2-4-13)22-7-8-27-11-17(22)24/h1-6,14H,7-11H2,(H,21,25)/t14-/m1/s1 | |
| Chemical Name | 5-chloro-N-[[(5R)-2-oxo-3-[4-(3-oxomorpholin-4-yl)phenyl]-1,3-oxazolidin-5-yl]methyl]thiophene-2-carboxamide | |
| Synonyms | BAY 59-7939 R-enantiomer; BAY59-7939; BAY-59-7939 R-isomer; Rivaroxaban; 5-R-Rivaroxaban; 5R-Rivaroxaban; trade name: Xarelto. | |
| HS Tariff Code | 2934.99.9001 | |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| ln Vitro | An oral direct Factor Xa (FXa) inhibitor called rivaroxaban (BAY 59-7939) is being developed for the treatment and prevention of venous and arterial thrombosis. Rivaroxaban inhibits prothrombinase activity (IC50 2.1 nM) and human FXa (Ki 0.4 nM) competitively with selectivity that is >10,000 times better than that of other serine proteases. In comparison to rat plasma (IC50 290 nM), human and rabbit plasma exhibit a more effective inhibition of endogenous FXa by rivaroxaban (IC50 21 nM). In human plasma, it exhibits anticoagulant properties, activating partial thromboplastin time at 0.69 μM and increasing prothrombin time (PT)[2]. | ||
| ln Vivo | A strong and specific direct FXa inhibitor with good oral absorption and in vivo action is rivaroxaban (BAY 59-7939)[1]. When given as an intravenous bolus prior to thrombus induction, rivaroxaban (BAY 59-7939) decreases thrombus formation (ED50 0.1 mg/kg), suppresses FXa, and dose-dependently prolongs PT. At the ED50, there is a small change in PT and FXa (1.8-fold increase and 32% inhibition, respectively). At a dosage of 0.3 mg/kg, which virtually completely blocks thrombus formation, rivaroxaban exhibits a moderate prolongation of PT (3.2±0.5-fold) and a suppression of FXa activity (65±3%)[2]. | ||
| Animal Protocol |
|
||
| References |
[1]. Discovery of the novel antithrombotic agent 5-chloro-N-({(5S)-2-oxo-3- [4-(3-oxomorpholin-4-yl)phenyl]-1,3-oxazolidin-5-yl}methyl)thiophene- 2-carboxamide (BAY 59-7939): an oral, direct factor Xa inhibitor. J Med Chem. 2005 Sep 22;48(19). [2]. In vitro and in vivo studies of the novel antithrombotic agent BAY 59-7939--an oral, direct Factor Xa inhibitor. J Thromb Haemost. 2005 Mar;3(3):514-21. |
Solubility Data
| Solubility (In Vitro) |
|
|||
| Solubility (In Vivo) |
Solubility in Formulation 1: 2.5 mg/mL (5.74 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), suspension solution; with sonication. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 2.5 mg/mL (5.74 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. Solubility in Formulation 3: 0.5% methylcellulose+0.2% Tween 80:5 mg/mL (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.2942 mL | 11.4710 mL | 22.9421 mL | |
| 5 mM | 0.4588 mL | 2.2942 mL | 4.5884 mL | |
| 10 mM | 0.2294 mL | 1.1471 mL | 2.2942 mL |