Physicochemical Properties
| Molecular Formula | C10H6CL2N2O2 |
| Molecular Weight | 257.07 |
| Exact Mass | 255.981 |
| CAS # | 1018-71-9 |
| PubChem CID | 13916 |
| Appearance | Light green to green solid powder |
| Density | 1.523g/cm3 |
| Boiling Point | 410.5ºC at 760mmHg |
| Flash Point | 202.1ºC |
| Vapour Pressure | 1.42E-06mmHg at 25°C |
| Index of Refraction | 1.65 |
| LogP | 4.419 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 1 |
| Heavy Atom Count | 16 |
| Complexity | 272 |
| Defined Atom Stereocenter Count | 0 |
| SMILES | ClC1C(C2C=CC=C(Cl)C=2[N+]([O-])=O)=CNC=1 |
| InChi Key | QJBZDBLBQWFTPZ-UHFFFAOYSA-N |
| InChi Code | InChI=1S/C10H6Cl2N2O2/c11-8-3-1-2-6(10(8)14(15)16)7-4-13-5-9(7)12/h1-5,13H |
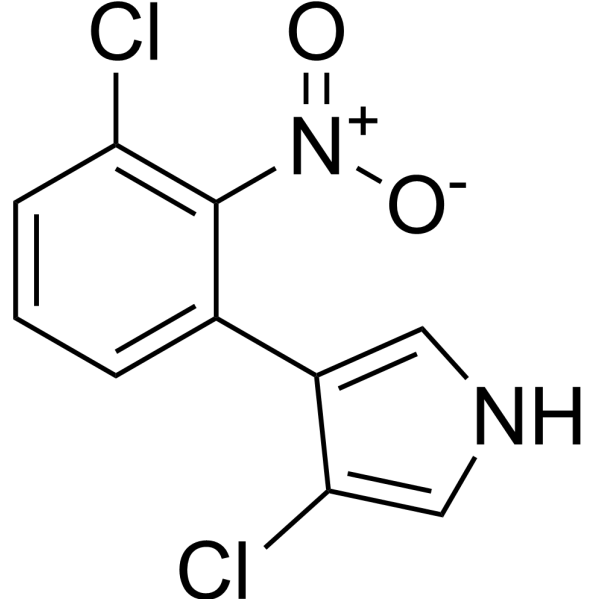
| Chemical Name | 3-chloro-4-(3-chloro-2-nitrophenyl)-1H-pyrrole |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | Target: fungi, yeast and gram-positive bacteria[1] |
| ln Vitro | At MIC values ranging from 0 to 100 μg/ml, pyrrolnitrin has antibiotic properties against a range of bacteria and fungi. With minimum inhibitory concentrations (MIC) of 50 μg/ml, 100 μg/ml, 0.78 μg/ml, 10 μg/ml, 12.5 μg/ml, and 1 μg/ml, respectively, it is effective against Staphylococcus aureus, Mycobacterium, Bacillus subtillis, Candida albicans, Aspergillus niger, and Trichophyton rubrum1. A microtitre plate experiment demonstrates the antibacterial effect of pyrrolnitrin (0-100 μg/ml). With MIC of 6.25 μg/ml, it is effective against Bacillus subtilis ATCC 6051, Bacillus licheniformis ATCC 14580, Bacillus coagulans ATCC 7050, and Bacillus thuringiensis ATCC 10792. |
| References |
[1]. K H van Pée, et al. Biosynthesis of Pyrrolnitrin and Other Phenylpyrrole Derivatives by Bacteria. Nat Prod Rep. 2000 Apr;17(2):157-64. [2]. N el-Banna, et al. Pyrrolnitrin From Burkholderia Cepacia: Antibiotic Activity Against Fungi and Novel Activities Against Streptomycetes.J Appl Microbiol. 1998 Jul;85(1):69-78. [3]. R S Gordee, et al.Systemic Antifungal Activity of Pyrrolnitrin.Appl Microbiol. 1969 May;17(5):690-4. |
| Additional Infomation |
Pyrrolnitrin is a member of the class of pyrroles carrying chloro and 3-chloro-2-nitrophenyl substituents at positions 3 and 4 respectively. It has a role as a bacterial metabolite and an antifungal drug. It is a member of pyrroles, a member of monochlorobenzenes, a C-nitro compound and an alkaloid. Pyrrolnitrin has been reported in Pseudomonas, Burkholderia cepacia, and other organisms with data available. Pyrrolnitrin is a pyrrole antifungal agent isolated from several Pseudomonas species. Pyrrolnitrin interrupts the terminal electron transport system and inhibits cellular respiration. This agent has activity against dermatophytic fungi, especially species of Trichophyton. 3-Chloro-4-(3-chloro-2-nitrophenyl)pyrrole. Antifungal antibiotic isolated from Pseudomonas pyrrocinia. It is effective mainly against Trichophyton, Microsporium, Epidermophyton, and Penicillium. |
Solubility Data
| Solubility (In Vitro) | May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples |
| Solubility (In Vivo) |
Note: Listed below are some common formulations that may be used to formulate products with low water solubility (e.g. < 1 mg/mL), you may test these formulations using a minute amount of products to avoid loss of samples. Injection Formulations (e.g. IP/IV/IM/SC) Injection Formulation 1: DMSO : Tween 80: Saline = 10 : 5 : 85 (i.e. 100 μL DMSO stock solution → 50 μL Tween 80 → 850 μL Saline) *Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH ₂ O to obtain a clear solution. Injection Formulation 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL DMSO → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Injection Formulation 3: DMSO : Corn oil = 10 : 90 (i.e. 100 μL DMSO → 900 μL Corn oil) Example: Take the Injection Formulation 3 (DMSO : Corn oil = 10 : 90) as an example, if 1 mL of 2.5 mg/mL working solution is to be prepared, you can take 100 μL 25 mg/mL DMSO stock solution and add to 900 μL corn oil, mix well to obtain a clear or suspension solution (2.5 mg/mL, ready for use in animals). Injection Formulation 4: DMSO : 20% SBE-β-CD in saline = 10 : 90 [i.e. 100 μL DMSO → 900 μL (20% SBE-β-CD in saline)] *Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Injection Formulation 5: 2-Hydroxypropyl-β-cyclodextrin : Saline = 50 : 50 (i.e. 500 μL 2-Hydroxypropyl-β-cyclodextrin → 500 μL Saline) Injection Formulation 6: DMSO : PEG300 : castor oil : Saline = 5 : 10 : 20 : 65 (i.e. 50 μL DMSO → 100 μLPEG300 → 200 μL castor oil → 650 μL Saline) Injection Formulation 7: Ethanol : Cremophor : Saline = 10: 10 : 80 (i.e. 100 μL Ethanol → 100 μL Cremophor → 800 μL Saline) Injection Formulation 8: Dissolve in Cremophor/Ethanol (50 : 50), then diluted by Saline Injection Formulation 9: EtOH : Corn oil = 10 : 90 (i.e. 100 μL EtOH → 900 μL Corn oil) Injection Formulation 10: EtOH : PEG300:Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL EtOH → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Oral Formulations Oral Formulation 1: Suspend in 0.5% CMC Na (carboxymethylcellulose sodium) Oral Formulation 2: Suspend in 0.5% Carboxymethyl cellulose Example: Take the Oral Formulation 1 (Suspend in 0.5% CMC Na) as an example, if 100 mL of 2.5 mg/mL working solution is to be prepared, you can first prepare 0.5% CMC Na solution by measuring 0.5 g CMC Na and dissolve it in 100 mL ddH2O to obtain a clear solution; then add 250 mg of the product to 100 mL 0.5% CMC Na solution, to make the suspension solution (2.5 mg/mL, ready for use in animals). Oral Formulation 3: Dissolved in PEG400 Oral Formulation 4: Suspend in 0.2% Carboxymethyl cellulose Oral Formulation 5: Dissolve in 0.25% Tween 80 and 0.5% Carboxymethyl cellulose Oral Formulation 6: Mixing with food powders Note: Please be aware that the above formulations are for reference only. InvivoChem strongly recommends customers to read literature methods/protocols carefully before determining which formulation you should use for in vivo studies, as different compounds have different solubility properties and have to be formulated differently. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.8900 mL | 19.4500 mL | 38.8999 mL | |
| 5 mM | 0.7780 mL | 3.8900 mL | 7.7800 mL | |
| 10 mM | 0.3890 mL | 1.9450 mL | 3.8900 mL |