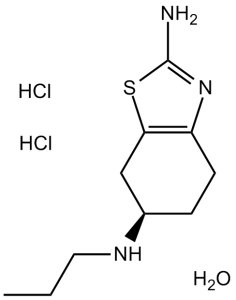
Pramipexole 2HCl monohydrate (formerly known as SND 919; (S)-Pramipexole; trade name Mirapex among others) is the dihydrochloride salt and hydrated form of of pramipexole which is a potent agonist of the Gαi-linked dopamine receptors D2, D3, and D4 with neuroprotective effects. Pramipexole is an innovative chemical dopamine agonist that is used to treat Parkinson's disease symptoms. It also has antioxidant properties and protects substantia nigral dopamine neurons in hypoxic-ischemic and methamphetamine models. Methylpyridinium ion (MPP+) produces oxygen radicals, and pramipexole lowers their levels when injected into rat striatum and incubated with SH-SY5Y cells. Additionally, pramipexole shows concentration-dependent inhibition of calcium and phosphate or MPP+-induced opening of the mitochondrial transition pore.
Physicochemical Properties
| Molecular Formula | C10H21CL2N3OS | |
| Molecular Weight | 302.26 | |
| Exact Mass | 301.078 | |
| Elemental Analysis | C, 39.74; H, 7.00; Cl, 23.46; N, 13.90; O, 5.29; S, 10.61 | |
| CAS # | 191217-81-9 | |
| Related CAS # | Pramipexole dihydrochloride; 104632-25-9; Dexpramipexole dihydrochloride; 104632-27-1; Pramipexole; 104632-26-0; Dexpramipexole; 104632-28-2 | |
| PubChem CID | 166589 | |
| Appearance | White to off-white solid powder | |
| Boiling Point | 378ºC at 760 mmHg | |
| Melting Point | 290 °C(dec.) | |
| Flash Point | 182.4ºC | |
| Vapour Pressure | 9.93E-11mmHg at 25°C | |
| LogP | 4.094 | |
| Hydrogen Bond Donor Count | 5 | |
| Hydrogen Bond Acceptor Count | 5 | |
| Rotatable Bond Count | 3 | |
| Heavy Atom Count | 17 | |
| Complexity | 188 | |
| Defined Atom Stereocenter Count | 1 | |
| SMILES | Cl[H].Cl[H].S1C(N([H])[H])=NC2=C1C([H])([H])[C@]([H])(C([H])([H])C2([H])[H])N([H])C([H])([H])C([H])([H])C([H])([H])[H].O([H])[H] |
|
| InChi Key | APVQOOKHDZVJEX-QTPLPEIMSA-N | |
| InChi Code | InChI=1S/C10H17N3S.2ClH.H2O/c1-2-5-12-7-3-4-8-9(6-7)14-10(11)13-8;;;/h7,12H,2-6H2,1H3,(H2,11,13);2*1H;1H2/t7-;;;/m0.../s1 | |
| Chemical Name | (6S)-6-N-propyl-4,5,6,7-tetrahydro-1,3-benzothiazole-2,6-diamine;hydrate;dihydrochloride | |
| Synonyms |
|
|
| HS Tariff Code | 2934.99.9001 | |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month Note: Please store this product in a sealed and protected environment, avoid exposure to moisture. |
|
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | D2 Receptor ( Ki = 3.9 nM ); D3 Receptor ( Ki = 0.5 nM ); D4 Receptor ( Ki = 1.3 nM ) | ||
| ln Vitro |
|
||
| ln Vivo |
|
||
| Cell Assay | Sporadic Parkinson's disease is associated with a defect in the activity of complex I of the mitochondrial electron transport chain. This electron transport chain defect is transmitted through mitochondrial DNA, and when expressed in host cells leads to increased oxygen free radical production, increased antioxidant enzyme activities, and increased susceptibility to programmed cell death. Pramipexole, a chemically novel dopamine agonist used for the treatment of Parkinson's disease symptoms, possesses antioxidant activity and is neuroprotective toward substantia nigral dopamine neurons in hypoxic-ischemic and methamphetamine models[1]. | ||
| Animal Protocol |
|
||
| Toxicity/Toxicokinetics |
Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation No information is available on the use of pramipexole during breastfeeding, but it suppresses serum prolactin and may interfere with breastfeeding. An alternate drug may be preferred, especially while nursing a newborn or preterm infant. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information in nursing mothers was not found as of the revision date. Pramipexole lowers serum prolactin.[1] The prolactin level in a mother with established lactation may not affect her ability to breastfeed. |
||
| References |
[1]. J Neurochem . 1998 Jul;71(1):295-301. [2]. Eur J Pharmacol . 1992 May 14;215(2-3):161-70. |
||
| Additional Infomation |
Pramipexole hydrochloride is a hydrate that is the monohydrate of the dihydrochloride salt of pramiprexole. It has a role as a dopamine agonist and an antiparkinson drug. It contains a member of pramipexole hydrochloride anhydrous and a pramipexole(2+). A benzothiazole derivative and dopamine agonist with antioxidant properties that is used in the treatment of PARKINSON DISEASE and RESTLESS LEGS SYNDROME. See also: Pramipexole (annotation moved to); Pramipexole Dihydrochloride (annotation moved to). Drug Indication Pramipexole Accord is indicated in adults for treatment of the signs and symptoms of idiopathic Parkinson's disease, alone (without levodopa) or in combination with levodopa, i. e. over the course of the disease, through to late stages when the effect of levodopa wears off or becomes inconsistent and fluctuations of the therapeutic effect occur (end-of-dose or 'on-off' fluctuations). DAQUIRAN tablets are indicated for treatment of the signs and symptoms of advanced idiopathic Parkinson's disease in combination with levodopa, i. e. over the course of the disease, when the effect of levodopa wears off or becomes inconsistent and fluctuations of the therapeutic effect occur (end of dose or " on off" fluctuations). Combined vocal and multiple motor tic disorder (de la Tourette), Restless Legs Syndrome Combined vocal and multiple motor tic disorder (de la Tourette), Restless Legs Syndrome |
Solubility Data
| Solubility (In Vitro) |
|
|||
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2 mg/mL (6.62 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 20.0 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 2 mg/mL (6.62 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 20.0 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 3: ≥ 2 mg/mL (6.62 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 20.0 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. Solubility in Formulation 4: 100 mg/mL (330.84 mM) in PBS (add these co-solvents sequentially from left to right, and one by one), clear solution; with ultrasonication. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.3084 mL | 16.5420 mL | 33.0841 mL | |
| 5 mM | 0.6617 mL | 3.3084 mL | 6.6168 mL | |
| 10 mM | 0.3308 mL | 1.6542 mL | 3.3084 mL |