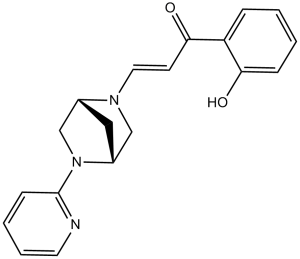
PFI-3 (PFI3) is a potent, selective, acetyl-lysine-competitive, and cell-permeable inhibitor of SMARCA bromodomains (SMARCA2/4 and PB1(5)) with antineoplastic activity. It inhibits SMARCA2/4 bromodomains with Kd values of 55 and 110 nM, respectively.
Physicochemical Properties
| Molecular Formula | C19H19N3O2 | |
| Molecular Weight | 321.37 | |
| Exact Mass | 321.147 | |
| CAS # | 1819363-80-8 | |
| Related CAS # |
|
|
| PubChem CID | 78243717 | |
| Appearance | Light yellow to green yellow solid powder | |
| Density | 1.3±0.1 g/cm3 | |
| Boiling Point | 528.5±50.0 °C at 760 mmHg | |
| Flash Point | 273.4±30.1 °C | |
| Vapour Pressure | 0.0±1.4 mmHg at 25°C | |
| Index of Refraction | 1.712 | |
| LogP | 2.19 | |
| Hydrogen Bond Donor Count | 1 | |
| Hydrogen Bond Acceptor Count | 5 | |
| Rotatable Bond Count | 4 | |
| Heavy Atom Count | 24 | |
| Complexity | 495 | |
| Defined Atom Stereocenter Count | 2 | |
| SMILES | C1[C@@H]2CN([C@H]1CN2C3=CC=CC=N3)/C=C/C(=O)C4=CC=CC=C4O |
|
| InChi Key | INAICWLVUAKEPB-QSTFCLMHSA-N | |
| InChi Code | InChI=1S/C19H19N3O2/c23-17-6-2-1-5-16(17)18(24)8-10-21-12-15-11-14(21)13-22(15)19-7-3-4-9-20-19/h1-10,14-15,23H,11-13H2/b10-8+/t14-,15-/m1/s1 | |
| Chemical Name | (E)-1-(2-hydroxyphenyl)-3-[(1R,4R)-5-pyridin-2-yl-2,5-diazabicyclo[2.2.1]heptan-2-yl]prop-2-en-1-one | |
| Synonyms |
|
|
| HS Tariff Code | 2934.99.9001 | |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| ln Vitro | PFI-3 is a potent, cell-permeable probe capable of displacing ectopically produced, GFP-tagged SMARCA2-bromodomain from chromatin. PFI-3 binds aggressively to both SMARCA2 and SMARCA4 bromodomains (BROMOScan Kd's between 55 and 110 nM) comparable with the binding constant (Kd=89 nM) observed by isothermal titration calorimetry. PFI-3 does not phenocopy the growth inhibitory effects of SMARCA2 knockdown in lung cancer[1]. Exposure of embryonic stem cells to PFI-3 leads to deprivation of stemness and deregulates lineage specification. Furthermore, differentiation of trophoblast stem cells in the presence of PFI-3 is considerably enhanced[2]. PFI-3 binds to some family VIII bromodomains while demonstrating significant, broader bromodomain family selectivity. The remarkable specificity of PFI-3 for family VIII is accomplished through a new bromodomain binding method of a phenolic headgroup that results to the unusual displacement of water molecules that are normally maintained by most other bromodomain inhibitors described to date[3]. | ||
| ln Vivo |
|
||
| Animal Protocol |
|
||
| References |
[1]. The SMARCA2/4 ATPase Domain Surpasses the Bromodomain as a Drug Target in SWI/SNF-Mutant Cancers: Insights from cDNA Rescue and PFI-3 Inhibitor Studies. Cancer Res. 2015 Sep 15;75(18):3865-78. [2]. Selective targeting of the BRG/PB1 bromodomains impairs embryonic and trophoblast stem cell maintenance. Sci Adv. 2015 Nov 13;1(10):e1500723. [3]. Identification of a Chemical Probe for Family VIII Bromodomains through Optimization of a Fragment Hit. J Med Chem. 2016 May 26;59(10):4800-11. |
||
| Additional Infomation | PFI-3 is an azabicycloalkane that is (1R,4R)-2,5-diazabicyclo[2.2.1]heptane which is substituted at position 2 by a 3-(2-hydroxyphenyl)-3-oxoprop-1-en-1-yl group and at position 5 by a pyridin-2-yl group. It is a potent and selective inhibitor of polybromo 1 (Kd = 48 nM), SMARCA2 and SMARCA4 (Kd = 89 nM) bromodomains. It is a member of pyridines, an azabicycloalkane, a member of phenols and an enone. |
Solubility Data
| Solubility (In Vitro) |
|
|||
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.25 mg/mL (7.00 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 22.5 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 2.25 mg/mL (7.00 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 22.5 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 3: ≥ 2.25 mg/mL (7.00 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 22.5 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.1117 mL | 15.5584 mL | 31.1168 mL | |
| 5 mM | 0.6223 mL | 3.1117 mL | 6.2234 mL | |
| 10 mM | 0.3112 mL | 1.5558 mL | 3.1117 mL |