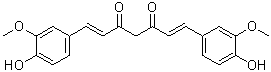
Curcumin (Diferuloylmethane; NSC32982; Turmeric Yellow; curcumin I) is a naturally occurring diarylheptanoid diarylheptanoid with diverse pharmacologic effects including anti-inflammatory, antioxidant, antiproliferative and antiangiogenic activities. It is the major curcuminoid of turmeric, a member of the ginger family (Zingiberaceae). Curcumin is an inhibitor of p300 histone acetylatransferase ((HATs)) and also shows inhibitory effects on NF-κB and MAPKs. Curcumin has the potential for treating various diseases, including multiple myeloma, pancreatic cancer, myelodysplastic syndromes, colon cancer, psoriasis, arthritis, major depressive disorder and Alzheimer's disease.
Physicochemical Properties
| Molecular Formula | C21H20O6 |
| Molecular Weight | 368.38 |
| Exact Mass | 368.125 |
| CAS # | 458-37-7 |
| Related CAS # | Curcumin-d6;1246833-26-0 |
| PubChem CID | 969516 |
| Appearance | Yellow to orange solid powder |
| Density | 1.3±0.1 g/cm3 |
| Boiling Point | 593.2±50.0 °C at 760 mmHg |
| Melting Point | 183 °C |
| Flash Point | 209.7±23.6 °C |
| Vapour Pressure | 0.0±1.8 mmHg at 25°C |
| Index of Refraction | 1.672 |
| LogP | 2.85 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 8 |
| Heavy Atom Count | 27 |
| Complexity | 507 |
| Defined Atom Stereocenter Count | 0 |
| SMILES | COC1=C(C=CC(=C1)/C=C/C(=O)CC(=O)/C=C/C2=CC(=C(C=C2)O)OC)O |
| InChi Key | VFLDPWHFBUODDF-FCXRPNKRSA-N |
| InChi Code | InChI=1S/C21H20O6/c1-26-20-11-14(5-9-18(20)24)3-7-16(22)13-17(23)8-4-15-6-10-19(25)21(12-15)27-2/h3-12,24-25H,13H2,1-2H3/b7-3+,8-4+ |
| Chemical Name | (1E,6E)-1,7-bis(4-hydroxy-3-methoxyphenyl)hepta-1,6-diene-3,5-dione |
| Synonyms | Turmeric Yellow NSC32982Diferuloylmethane NSC-32982Curcumincurcumin I C.I. 75300 Natural Yellow 3 |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| ln Vitro | Part of how curcumin works as a chemopreventive agent is via activating its antioxidant and phase II detoxifying enzymes, as well as nuclear factor (erythroid-2 related) factor 2 (Nrf2)[1]. With IC50s of 25, 19, and 17.5 μM for 24, 48, and 72-hour MTT experiments, respectively, curcumin suppresses the proliferation of T47D cells. For 24, 48, and 72 hours of exposure, the IC50s of the curcumin and silibinin mixture against T47D cells are 17.5, 15, and 12 μM, respectively[2]. AGS and HT-29 cell lines exhibit apoptotic cell death in response to curcumin (2.5-80 μM); the IC50 values for these cell lines are 21.9±0.1 and 40.7±0.5 μM, respectively. In AGS and HT-29 cells, caspase activity are necessary for curcumin-induced apoptosis. Curcumin causes mitochondrial Ca2+ overloading and ER Ca2+ decline[3]. Curcumin dose-dependently promotes LNCaP and PC-3 cells to enter the G2/M cell cycle arrest. Curcumin decreases the protein levels of c-Jun and AR while increasing the protein level of the NF-kappaB inhibitor IkappaBalpha[5]. | |
| ln Vivo | Compared to the rats exposed to CMS, curcumin (10 mg/kg, po) significantly avoids declines in the percentage of sucrose consumption. When stressed rats are treated with curcumin, their levels of TNF-α and IL-6 are significantly prevented from rising[4]. In chronic constriction injury (CCI) rats, curcumin reduces the binding of p300/CREB-binding protein (CBP) at the brain-derived neurotrophic factor (BDNF) promoter at 20 mg/kg (ip), as well as the binding of P300/CBP at 40 mg/kg and the binding of all four proteins of p300/CBP and H3K9ac/H4K5ac at 60 mg/kg[6]. | |
| Cell Assay |
|
|
| ADME/Pharmacokinetics |
Absorption, Distribution and Excretion Curcumin displays poor absorption into the gastrointestinal tract. In a rat study, oral administration of a single dose of 2 g of curcumin resulted in a plasma concentration of less than 5 μg/mL, indicating poor absorption from the gut. Following oral administration of curcumin to rats at a dose of 1 g/kg bw, about 75% of dose was excreted in the faeces and only traces of the compound was detected in the urine. When a single 400 mg dose of curcumin was administered orally to rats, about 60% was absorbed and 40% was excreted unchanged in the faeces over an period of 5 days. Intraperitoneal administration resulted in fecal excretion of 73% and biliary excretion of 11%. Following oral administration of radio-labelled curcumin to rats, radioactivity was detected in the liver and kidneys. No pharmacokinetic data available. Oral & ip doses of (3)H-curcumin led to fecal excretion of most of radioactivity. Iv & ip doses were well excreted in bile of cannulated rats. When admin orally in dose of 1 g/kg, curcumin was excreted in feces to about 75%, while negligible amt appeared in urine. Measurement of blood plasma levels & biliary excretion showed that curcumin was poorly absorbed from the gut. The aim of this study was to develop a curcumin intranasal thermosensitive hydrogel and to improve its brain targeting efficiency. The hydrogel gelation temperature, gelation time, drug release and mucociliary toxicity characteristics as well as the nose-to-brain transport in the rat model were evaluated. The developed nasal hydrogel, composed of Pluronic F127 and Poloxamer 188, had shorter gelation time, longer mucociliary transport time and produced prolonged curcumin retention in the rat nasal cavity at body temperature. The hydrogel release mechanism was diffusion-controlled drug release, evaluated by the dialysis membrane method, but dissolution-controlled release when evaluated by the membraneless method. A mucociliary toxicity study revealed that the hydrogel maintained nasal mucosal integrity until 14 days after application. The drug-targeting efficiencies for the drug in the cerebrum, cerebellum, hippocampus and olfactory bulb after intranasal administration of the curcumin hydrogel were 1.82, 2.05, 2.07 and 1.51 times that after intravenous administration of the curcumin solution injection, respectively, indicating that the hydrogel significantly increased the distribution of curcumin into the rat brain tissue, especially into the cerebellum and hippocampus. A thermosensitive curcumin nasal gel was developed with favourable gelation, release properties, biological safety and enhanced brain-uptake efficiency. /Curcumin intranasal thermosensitive hydrogel/ ...However, curcumin has a low systemic bioavailability, so it is imperative to improve the bioavailability of curcumin in its clinical application. Many methods, such as adjuvant drug delivery system and structural modification have been demonstrated to have a potential effect. For more Absorption, Distribution and Excretion (Complete) data for CURCUMIN (6 total), please visit the HSDB record page. Metabolism / Metabolites Initially, curcumin undergoes rapid intestinal metabolism to form curcumin glucuronide and curcumin sulfate via O-conjugation. Other metabolites formed include tetrahydrocurcumin, hexahydrocurcumin, and hexahydrocurcuminol via reduction. Curcumin may also undergo intensive second metabolism in the liver where the major metabolites were glucuronides of tetrahydrocurcumin and hexahydrocurcumin, with dihydroferulic acid and traces of ferulic acid as further metabolites. Hepatic metabolites are expected to be excreted in the bile. Certain curcumin metabolites, such as tetrahydrocurcumin, retain anti-inflammatory and antioxidant properties. Iv & ip doses of (3)H-curcumin excreted in bile of cannulated rats. Major metab were glucuronides of tetrahydrocurcumin & hexahydrocurcumin. Minor metab was dihydroferulic acid together with traces of ferulic acid. Curcumin has known human metabolites that include Curcumin 4-O-glucuronide and O-demethyl curcumin. Biological Half-Life No pharmacokinetic data available. |
|
| Toxicity/Toxicokinetics |
Interactions Groundwater arsenic contamination has been a health hazard for West Bengal, India. Oxidative stress to DNA is recognized as an underlying mechanism of arsenic carcinogenicity. A phytochemical, curcumin, from turmeric appears to be potent antioxidant and antimutagenic agent. DNA damage prevention with curcumin could be an effective strategy to combat arsenic toxicity. This field trial in Chakdah block of West Bengal evaluated the role of curcumin against the genotoxic effects of arsenic. DNA damage in human lymphocytes was assessed by comet assay and fluorescence-activated DNA unwinding assay. Curcumin was analyzed in blood by high performance liquid chromatography (HPLC). Arsenic induced oxidative stress and elucidation of the antagonistic role of curcumin was done by observation on reactive oxygen species (ROS) generation, lipid peroxidation and protein carbonyl. Antioxidant enzymes like catalase, superoxide dismutase, glutathione reductase, glutathioneS-transferase, glutathione peroxidase and non-enzymatic glutathione were also analyzed. The blood samples of the endemic regions showed severe DNA damage with increased levels of ROS and lipid peroxidation. The antioxidants were found with depleted activity. Three months curcumin intervention reduced the DNA damage, retarded ROS generation and lipid peroxidation and raised the level of antioxidant activity. Thus curcumin may have some protective role against the DNA damage caused by arsenic. To determine whether curcumin ameliorates acute and chronic radiation skin toxicity and to examine the expression of inflammatory cytokines (interleukin [IL]-1, IL-6, IL-18, IL-1Ra, tumor necrosis factor [TNF]-alpha, and lymphotoxin-beta) or fibrogenic cytokines (transforming growth factor [TGF]-beta) during the same acute and chronic phases. Curcumin was given intragastrically or intraperitoneally to C3H/HeN mice either: 5 days before radiation; 5 days after radiation; or both 5 days before and 5 days after radiation. The cutaneous damage was assessed at 15-21 days (acute) and 90 days (chronic) after a single 50 Gy radiation dose was given to the hind leg. Skin and muscle tissues were collected for measurement of cytokine mRNA. Curcumin, administered before or after radiation, markedly reduced acute and chronic skin toxicity in mice (p < 0.05). Additionally, curcumin significantly decreased mRNA expression of early responding cytokines (IL-1 IL-6, IL-18, TNF-alpha, and lymphotoxin-beta) and the fibrogenic cytokine, TGF-beta, in cutaneous tissues at 21 days postradiation. Curcumin has a protective effect on radiation-induced cutaneous damage in mice, which is characterized by a downregulation of both inflammatory and fibrogenic cytokines in irradiated skin and muscle, particularly in the early phase after radiation. These results may provide the molecular basis for the application of curcumin in clinical radiation therapy. The aim of this study is to evaluate the effect of curcumin in protecting against selenium-induced toxicity in liver and kidney of Wistar rats. Light microscopy evaluation of selenium alone administered rats showed liver to be infiltrated with mononuclear cells, vacuolation, necrosis, and pronounced degeneration. Control liver sections showed a regular morphology of parenchymal cells with intact hepatocytes and sinusoids. Kidney from selenium alone administered rats showed vacuolar degeneration changes in the epithelial cells, cellular proliferation with fibrosis, thickening of capillary walls, and glomerular tuft atrophy. Such changes were also observed in rats administered with selenium and curcumin simultaneously and rats administered first with selenium and then curcumin 24 hr later. Interestingly, such degenerative changes observed in liver and kidney induced by selenium were not seen in rats that were administered with curcumin first and selenium 24 hr later. This clearly suggests the protective nature of curcumin against selenium toxicity. To understand the probable mechanism of action of curcumin, /investigators/ analyzed inducible nitric oxide synthase (iNOS) expression by immunohistochemistry, and the results showed an increased iNOS expression in selenium-alone induced liver and kidney. Such high iNOS levels were inhibited in liver and kidney of rats pretreated with curcumin and then with selenium 24 hr later. Based on the histological results, it can be concluded that curcumin functions as a protective agent against selenium-induced toxicity in liver as well as kidney, and this action is probably by the regulatory role of curcumin on iNOS expression. Zn(II)-curcumin, a mononuclear (1:1) zinc complex of curcumin was synthesized and examined for its antiulcer activities against pylorus-ligature-induced gastric ulcer in rats. The structure of Zn(II)-curcumin was identified by elemental analysis, NMR and TG-DTA analysis. It was found that a zinc atom was coordinated through the keto-enol group of curcumin along with one acetate group and one water molecule. Zn(II)-curcumin (12, 24 and 48 mg/kg) dose-dependently blocked gastric lesions, significantly reduced gastric volume, free acidity, total acidity and pepsin, compared with control group (P<0.001) and curcumin alone (24 mg/kg, P<0.05). Reverse transcriptase polymerase chain reaction (RT-PCR) analysis showed that Zn(II)-curcumin markedly inhibited the induction of nuclear factor-kappa B (NF-kappaB), transforming growth factor beta(1) (TGF-beta(1)) and interleukin-8 (IL-8), compared with control group (P<0.05). These findings suggested that Zn(II)-curcumin prevented pylorus-ligation-induced lesions in rat by inhibiting NF-kappaB activation and the subsequent production of proinflammatory cytokines, indicating a synergistic effect between curcumin and zinc.../Zinc(II)-curcumin complex/ For more Interactions (Complete) data for CURCUMIN (12 total), please visit the HSDB record page. Non-Human Toxicity Values LD50 Mice oral more than 2000 mg/kg bw /Solid lipid curcumin particle/ LD50 Rats oral more than 2000 mg/kg bw /Solid lipid curcumin particle/ LD50 Zebrafish embryo 7.5 uM (24 hr); LD50 Zebrafish larvae 5 uM (24 hr) LD50 Mice oral more than 2000 mg/kg LD50 Rats oral more than 5000 mg/kg /Curcumin oil/ |
|
| References |
[1]. Curcumin attenuates arsenic-induced hepatic injuries and oxidative stress in experimental mice through activation of Nrf2 pathway, promotion of arsenic methylation and urinary excretion. Food Chem Toxicol. 2013 Jul 18. pii: S0278-6915(13)004. [2]. Curcumin and Silibinin Inhibit Telomerase Expression in T47D Human Breast Cancer Cells. Asian Pac J Cancer Prev. 2013;14(6):3449-53. [3]. Cao A, et all. Curcumin induces apoptosis in human gastric carcinoma AGS cells and colon carcinoma HT-29 cells through mitochondrial dysfunction and endoplasmic reticulum stress. Apoptosis. 2013 Jul 24. [Epub ahead of print]. [4]. Antidepressant-like effects of curcumin in chronic mild stress of rats: Involvement of its anti-inflammatory action. Prog Neuropsychopharmacol Biol Psychiatry. 2013 Jul 20. pii: S0278-5846(13)00150-4. [5]. Curcumin induces cell cycle arrest and apoptosis of prostate cancer cells by regulating the expression of IkappaBalpha, c-Jun and androgen receptor. Pharmazie. 2013 Jun;68(6):431-4. [6]. Curcumin alleviates neuropathic pain by inhibiting p300/CBP histone acetyltransferase activity-regulated expression of BDNF and cox-2 in a rat model. PLoS One. 2014 Mar 6;9(3):e91303. [7]. Curcumin, a novel p300/CREB-binding protein-specific inhibitor of acetyltransferase, represses the acetylation of histone/nonhistone proteins and histone acetyltransferase-dependent chromatin transcription. J Biol Chem. 2004 Dec 3;279(49):51163-71. [8]. Curcumin induces stabilization of Nrf2 protein through Keap1 cysteine modification. Biochem Pharmacol. 2020 Mar;173:113820. |
|
| Additional Infomation |
Therapeutic Uses EXPL THER Curcumin (Cum) has been reported to have potential chemo-preventive and chemotherapeutic activity through influencing various processes, inducing cell cycle arrest, differentiation and apoptosis in a series of cancers. However, the poor solubility of Cum limits its further applications in the treatment of cancer. /The authors/ have previously reported Cum-loaded nanoparticles (Cum-NPs) prepared with amphilic methoxy poly(ethylene glycol)-polycaprolactone (mPEG-PCL) block copolymers. The current study demonstrated superior antitumor efficacy of Cum-NPs over free Cum in the treatment of lung cancer. In vivo evaluation further demonstrated superior anticancer effects of Cum-NPs by delaying tumor growth compared to free Cum in an established A549 transplanted mice model. Moreover, Cum-NPs showed little toxicity to normal tissues including bone marrow, liver and kidney at a therapeutic dose. These results suggest that Cum-NPs are effective to inhibit the growth of human lung cancer with little toxicity to normal tissues, and could provide a clinically useful therapeutic regimen. They thus merit more research to evaluate the feasibility of clinical application./ Curcumin-loaded nanoparticles/ EXPL THER Accumulation of amyloid peptide (Abeta) in senile plaques is a hallmark lesion of Alzheimer disease (AD). The design of molecules able to target the amyloid pathology in tissue is receiving increasing attention, both for diagnostic and for therapeutic purposes. Curcumin is a fluorescent molecule with high affinity for the Abeta peptide but its low solubility limits its clinical use. Curcumin-conjugated nanoliposomes, with curcumin exposed at the surface, were designed. They appeared to be monodisperse and stable. They were non-toxic in vitro, down-regulated the secretion of amyloid peptide and partially prevented Abeta -induced toxicity. They strongly labeled Abeta deposits in post-mortem brain tissue of AD patients and APPxPS1 mice. Injection in the hippocampus and in the neocortex of these mice showed that curcumin-conjugated nanoliposomes were able to specifically stain the Abeta deposits in vivo. Curcumin-conjugated nanoliposomes could find application in the diagnosis and targeted drug delivery in AD. In this preclinical study, curcumin-conjugated nanoliposomes were investigated as possible diagnostics and targeted drug delivery system in Alzheimer's disease, demonstrating strong labeling of Abeta deposits both in human tissue and in mice, and in vitro downregulation of amyloid peptide secretion and prevention of Abeta -induced toxicity./ Curcumin-conjugated nanoliposomes/ EXPL THER The anti-inflammatory agent curcumin can selectively eliminate malignant rather than normal cells. The present study examined the effects of curcumin on the Lewis lung carcinoma (LLC) cell line and characterized a subpopulation surviving curcumin treatments. Cell density was measured after curcumin was applied at concentrations between 10 and 60 uM for 30 hours. Because of the high cell loss at 60 uM, this dose was chosen to select for surviving cells that were then used to establish a new cell line. The resulting line had approximately 20% slower growth than the original LLC cell line and based on ELISA contained less of two markers, NF-kB and ALDH1A, used to identify more aggressive cancer cells. /The authors/ also injected cells from the original and surviving lines subcutaneously into syngeneic C57BL/6 mice and monitored tumor development over three weeks and found that the curcumin surviving-line remained tumorigenic. Because curcumin has been reported to kill cancer cells more effectively when administered with light, /the authors/ examined this as a possible way of enhancing the efficacy of curcumin against LLC cells. When LLC cells were exposed to curcumin and light from a fluorescent lamp source, cell loss caused by 20 uM curcumin was enhanced by about 50%, supporting a therapeutic use of curcumin in combination with white light. This study is the first to characterize a curcumin-surviving subpopulation among lung cancer cells. It shows that curcumin at a high concentration either selects for an intrinsically less aggressive cell subpopulation or generates these cells. The findings further support a role for curcumin as an adjunct to traditional chemical or radiation therapy of lung and other cancers. EXPL THER 5-Fluorouracil (5-FU) is the first rationally designed antimetabolite, which achieves its therapeutic efficacy through inhibition of the enzyme thymidylate synthase (TS), which is essential for the synthesis and repair of DNA. However, prolonged exposure to 5-FU induces TS overexpression, which leads to 5-FU resistance in cancer cells. Several studies have identified curcumin as a potent chemosensitizer against chemoresistance induced by various chemotherapeutic drugs. In this study, /investigators/ report for the first time, with mechanism-based evidences, that curcumin can effectively chemosensitize breast cancer cells to 5-FU, thereby reducing the toxicity and drug resistance. /The authors/ found that 10 uM 5-FU and 10 uM curcumin induces a synergistic cytotoxic effect in different breast cancer cells, independent of their receptor status, through the enhancement of apoptosis. Curcumin was found to sensitize the breast cancer cells to 5-FU through TS-dependent downregulation of nuclear factor-kB (NF-kB), and this observation was confirmed by silencing TS and inactivating NF-kB, both of which reduced the chemosensitizing efficacy of curcumin. Silencing of TS suppressed 5-FU-induced NF-kB activation, whereas inactivation of NF-kB did not affect 5-FU-induced TS upregulation, confirming that TS is upstream of NF-kB and regulates the activation of NF-kB in 5-FU-induced signaling pathway. Although Akt/PI3kinase and mitogen-activated protein kinase pathways are activated by 5-FU and downregulated by curcumin, they do not have any role in regulating the synergism. As curcumin is a pharmacologically safe and cost-effective compound, its use in combination with 5-FU may improve the therapeutic index of 5-FU, if corroborated by in vivo studies and clinical trials. For more Therapeutic Uses (Complete) data for CURCUMIN (23 total), please visit the HSDB record page. Pharmacodynamics Intravenous application of 25 mg/kg bw curcumin to rats resulted in an increase in bile flow by 80 and 120%. In the rat model of inflammation, curcumin was shown to inhibit edema formation. In nude mouse that had been injected subcutaneously with prostate cancer cells, administration of curcumin caused a marked decrease in the extent of cell proliferation, a significant increase of apoptosis and micro-vessel density. Curcumin may exert choleretic effects by increasing biliary excretion of bile salts, cholesterol, and bilirubin, as well as increasing bile solubility. Curcumin inhibited arachidonic acid-induced platelet aggregation _in vitro_. |
Solubility Data
| Solubility (In Vitro) | DMSO : ~100 mg/mL (~271.46 mM) |
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 3 mg/mL (8.14 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 30.0 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: 3 mg/mL (8.14 mM) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), suspension solution; with ultrasonication. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 30.0 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 3: 25 mg/mL (67.86 mM) in 1% (w/v) carboxymethylcellulose (CMC) (add these co-solvents sequentially from left to right, and one by one), Suspension solution; with ultrasonication. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.7146 mL | 13.5729 mL | 27.1459 mL | |
| 5 mM | 0.5429 mL | 2.7146 mL | 5.4292 mL | |
| 10 mM | 0.2715 mL | 1.3573 mL | 2.7146 mL |