Olumacostat glasaretil (OG) is a novel and potent acetyl-CoA carboxylase inhibitor with anti-inflammatory effects. Acetyl-COA carboxylase is the key enzyme for the first, rate-limiting step in de novo fatty acid synthesis. Olumacostat glasaretil can inhibit in vitro human sebocyte lipid production, it can also reduce in vivo sebaceous gland size in hamster ears.
Physicochemical Properties
| Molecular Formula | C26H43NO7 |
| Molecular Weight | 481.63 |
| Exact Mass | 481.303 |
| Elemental Analysis | C, 64.84; H, 9.00; N, 2.91; O, 23.25 |
| CAS # | 1261491-89-7 |
| PubChem CID | 89497391 |
| Appearance | White to light yellow solid powder |
| Density | 1.1±0.1 g/cm3 |
| Boiling Point | 583.9±50.0 °C at 760 mmHg |
| Flash Point | 307.0±30.1 °C |
| Vapour Pressure | 0.0±1.6 mmHg at 25°C |
| Index of Refraction | 1.485 |
| LogP | 7.72 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 22 |
| Heavy Atom Count | 34 |
| Complexity | 569 |
| Defined Atom Stereocenter Count | 0 |
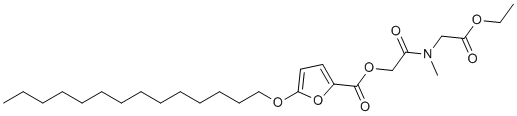
| SMILES | O(C1=C([H])C([H])=C(C(=O)OC([H])([H])C(N(C([H])([H])[H])C([H])([H])C(=O)OC([H])([H])C([H])([H])[H])=O)O1)C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])[H] |
| InChi Key | FYJLDICZGDFWKP-UHFFFAOYSA-N |
| InChi Code | InChI=1S/C26H43NO7/c1-4-6-7-8-9-10-11-12-13-14-15-16-19-32-25-18-17-22(34-25)26(30)33-21-23(28)27(3)20-24(29)31-5-2/h17-18H,4-16,19-21H2,1-3H3 |
| Chemical Name | [2-[(2-ethoxy-2-oxoethyl)-methylamino]-2-oxoethyl] 5-tetradecoxyfuran-2-carboxylate |
| Synonyms | DRM-01B DRM01B Olumacostat glasaretil |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| ln Vitro | The acetyl coenzyme One rate-limiting step in the production of fatty acids is controlled by a carboxylase. The de novo lipid synthesis in both primary and modified human sebocytes is inhibited by olumacostat glasaretil. Olumbacostat glasaretil lowers fatty acid synthesis to baseline levels or less at 3 μM. For SEB-1 cells treated with olumacostat glasaretil at 20 μM, 14C-acetate incorporation levels are 85%-90% lower than those of control samples. Lumacostat glasaretil, at 3 μM, decreases control values in sebocyte triacylglycerol, cholesteryl/wax ester, diacylglycerol, cholesterol, and phospholipid by, on average, 86%, 57%, 51%, 39%, and 37%, respectively[1]. | |
| ln Vivo | Olumacostat glasaretil is a pro-drug intended to improve in vivo administration of 5-(tetradecyloxy)-2-furoic acid (TOFA), an ACC inhibitor. The size of the sebaceous glands in hamster ears is greatly reduced by topical application of olumacostat glasaretil, but not TOFA. The olumacostat glasaretil administration raises ACC levels and the ratio of acetyl-CoA to free CoA in the studied animals, indicating accelerated fatty acid oxidation, according to HPLC analysis of hamster ear extracts. These modifications align with the inhibition of ACC. In comparison to the surrounding dermis, OG administered to Yorkshire pig ears accumulates in sebaceous glands, according to matrix-assisted laser desorption/ionization (MALDI) imaging[1]. By week 12, patients receiving OG therapy had more patients with investigator global evaluation score improvements of at least two grades and more decreases in both inflammatory and noninflammatory lesions from baseline[2]. | |
| Cell Assay |
|
|
| References |
[1]. Inhibition of Sebum Production with the Acetyl Coenzyme A Carboxylase Inhibitor OlumacostatGlasaretil. J Invest Dermatol. 2017 Mar 1. pii: S0022-202X(17)30186-0. [2]. Olumacostat glasaretil, a novel topical sebum inhibitor, in the treatment of acne vulgaris: A phase IIa, multicenter, randomized, vehicle-controlled study. J Am Acad Dermatol. 2017 Jan;76(1):33-39. |
|
| Additional Infomation | Olumacostat glasaretil has been used in trials studying the treatment of Acne Vulgaris. |
Solubility Data
| Solubility (In Vitro) | DMSO : ~125 mg/mL (~259.54 mM) |
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.08 mg/mL (4.32 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 20.8 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: 2.08 mg/mL (4.32 mM) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), suspension solution; with ultrasonication. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 20.8 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 3: ≥ 2.08 mg/mL (4.32 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 20.8 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.0763 mL | 10.3814 mL | 20.7628 mL | |
| 5 mM | 0.4153 mL | 2.0763 mL | 4.1526 mL | |
| 10 mM | 0.2076 mL | 1.0381 mL | 2.0763 mL |