Physicochemical Properties
| Molecular Formula | C20H18CL2N4O4 |
| Molecular Weight | 449.29 |
| Exact Mass | 448.07051 |
| Related CAS # | O-Demethyl Lenvatinib;417717-04-5 |
| PubChem CID | 168007119 |
| Appearance | Light yellow to yellow solid powder |
| InChi Key | KMZOGLZCTDVLLW-UHFFFAOYSA-N |
| InChi Code | InChI=1S/C20H17ClN4O4.ClH/c21-14-7-11(3-4-15(14)25-20(28)24-10-1-2-10)29-18-5-6-23-16-9-17(26)13(19(22)27)8-12(16)18;/h3-10,26H,1-2H2,(H2,22,27)(H2,24,25,28);1H |
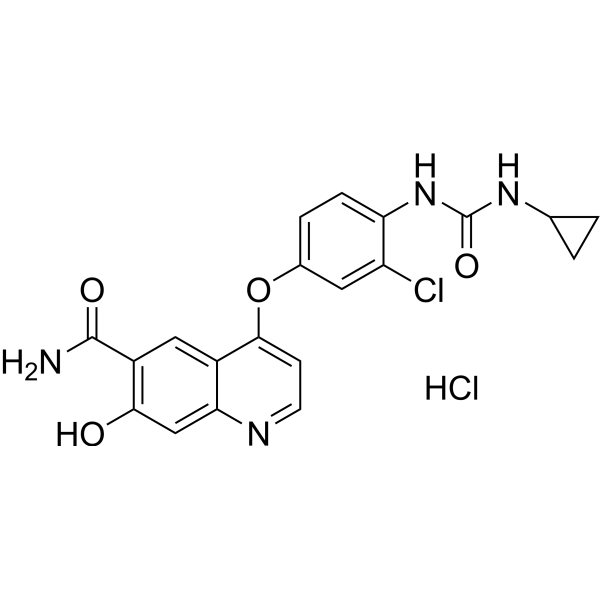
| Chemical Name | 4-[3-chloro-4-(cyclopropylcarbamoylamino)phenoxy]-7-hydroxyquinoline-6-carboxamide;hydrochloride |
| Synonyms | O-Demethyl Lenvatinib (hydrochloride); O-Demethyl Lenvatinib hydrochloride; |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month Note: Please store this product in a sealed and protected environment (e.g. under nitrogen), avoid exposure to moisture and light. |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | Lenvatinib metabolite |
| References |
[1]. Lenvatinib: A Promising Molecular Targeted Agent for Multiple Cancers. Cancer Control. 2018 Jan-Dec;25(1):1073274818789361. [2]. Lenvatinib versus Bay 43-9006 in first-line treatment of patients with unresectable hepatocellularcarcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018 Mar 24;391(10126):1163-1173. |
| Additional Infomation |
Lenvatinib is a small-molecule tyrosine kinase inhibitor that inhibits vascular endothelial growth factor receptor (VEGFR1-3), fibroblast growth factor receptor (FGFR1-4), platelet-derived growth factor receptor α (PDGFRα), stem cell factor receptor (KIT), and rearranged during transfection (RET). These receptors are important for tumor angiogenesis, and lenvatinib inhibits tumor angiogenesis by inhibiting function of these receptors. Phase I trials of lenvatinib were conducted at the same time in Japan, Europe, and the United States, and tumor shrinkage effects were observed in thyroid cancer, endometrial cancer, melanoma, renal cell carcinoma, sarcoma, and colon cancer. Lenvatinib is a promising drug that has shown therapeutic effects against various solid tumors. Adverse events, such as hypertension, proteinuria, diarrhea, and delayed wound healing, can occur with lenvatinib treatment. Managing these adverse events is also important for the use of lenvatinib. In this mini-review article, we outline the current state, toxicity, and future prospects of lenvatinib toward thyroid cancer, hepatocellular carcinoma, renal cell carcinoma, and lung cancer. [1] Background: In a phase 2 trial, lenvatinib, an inhibitor of VEGF receptors 1-3, FGF receptors 1-4, PDGF receptor α, RET, and KIT, showed activity in hepatocellular carcinoma. We aimed to compare overall survival in patients treated with lenvatinib versus sorafenib as a first-line treatment for unresectable hepatocellular carcinoma. Methods: This was an open-label, phase 3, multicentre, non-inferiority trial that recruited patients with unresectable hepatocellular carcinoma, who had not received treatment for advanced disease, at 154 sites in 20 countries throughout the Asia-Pacific, European, and North American regions. Patients were randomly assigned (1:1) via an interactive voice-web response system-with region; macroscopic portal vein invasion, extrahepatic spread, or both; Eastern Cooperative Oncology Group performance status; and bodyweight as stratification factors-to receive oral lenvatinib (12 mg/day for bodyweight ≥60 kg or 8 mg/day for bodyweight <60 kg) or sorafenib 400 mg twice-daily in 28-day cycles. The primary endpoint was overall survival, measured from the date of randomisation until the date of death from any cause. The efficacy analysis followed the intention-to-treat principle, and only patients who received treatment were included in the safety analysis. The non-inferiority margin was set at 1·08. The trial is registered with ClinicalTrials.gov, number NCT01761266. Findings: Between March 1, 2013 and July 30, 2015, 1492 patients were recruited. 954 eligible patients were randomly assigned to lenvatinib (n=478) or sorafenib (n=476). Median survival time for lenvatinib of 13·6 months (95% CI 12·1-14·9) was non-inferior to sorafenib (12·3 months, 10·4-13·9; hazard ratio 0·92, 95% CI 0·79-1·06), meeting criteria for non-inferiority. The most common any-grade adverse events were hypertension (201 [42%]), diarrhoea (184 [39%]), decreased appetite (162 [34%]), and decreased weight (147 [31%]) for lenvatinib, and palmar-plantar erythrodysaesthesia (249 [52%]), diarrhoea (220 [46%]), hypertension (144 [30%]), and decreased appetite (127 [27%]) for sorafenib. Interpretation: Lenvatinib was non-inferior to sorafenib in overall survival in untreated advanced hepatocellular carcinoma. The safety and tolerability profiles of lenvatinib were consistent with those previously observed.[2] |
Solubility Data
| Solubility (In Vitro) | DMSO :~50 mg/mL (~111.29 mM) |
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 1.8 mg/mL (4.01 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 18.0 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.2257 mL | 11.1287 mL | 22.2573 mL | |
| 5 mM | 0.4451 mL | 2.2257 mL | 4.4515 mL | |
| 10 mM | 0.2226 mL | 1.1129 mL | 2.2257 mL |