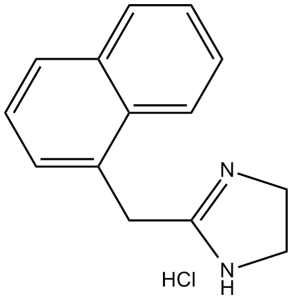
Naphazoline HCl (Albalon Liquifilm; Nafazair; Rhinantin; Vasocon), the hydrochloride salt of Naphazoline, is an imidazoline-based ocular vasoconstrictor and sympathomimetic amine used as a nasal decongestant.
Physicochemical Properties
| Molecular Formula | C14H15CLN2 | |
| Molecular Weight | 246.74 | |
| Exact Mass | 246.092 | |
| Elemental Analysis | C, 68.15; H, 6.13; Cl, 14.37; N, 11.35 | |
| CAS # | 550-99-2 | |
| Related CAS # | Naphazoline nitrate; 5144-52-5; Naphazoline; 835-31-4 | |
| PubChem CID | 11079 | |
| Appearance | White to off-white solid powder | |
| Density | 1.15 g/cm3 | |
| Boiling Point | 440.5ºC at 760 mmHg | |
| Melting Point | 254-260 °C | |
| Flash Point | 220.2ºC | |
| LogP | 2.95 | |
| Hydrogen Bond Donor Count | 2 | |
| Hydrogen Bond Acceptor Count | 1 | |
| Rotatable Bond Count | 2 | |
| Heavy Atom Count | 17 | |
| Complexity | 272 | |
| Defined Atom Stereocenter Count | 0 | |
| SMILES | C1(CC2=NCCN2)=CC=CC3=CC=CC=C13.Cl |
|
| InChi Key | DJDFFEBSKJCGHC-UHFFFAOYSA-N | |
| InChi Code | InChI=1S/C14H14N2.ClH/c1-2-7-13-11(4-1)5-3-6-12(13)10-14-15-8-9-16-14;/h1-7H,8-10H2,(H,15,16);1H | |
| Chemical Name | 2-(naphthalen-1-ylmethyl)-4,5-dihydro-1H-imidazole;hydrochloride | |
| Synonyms |
|
|
| HS Tariff Code | 2934.99.9001 | |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month Note: Please store this product in a sealed and protected environment, avoid exposure to moisture. |
|
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | IL-1β; IL-6; IL-4 |
| ln Vivo | Naphazoline hydrochloride (0.2 mg/kg, 10 µl per eye; IP, once) inhibits conjunctivitis in mice by reducing inflammation, NGF, and VEGF, as well as histamine- or antigen-induced conjunctival vascular hyperpermeability[1]. |
| Animal Protocol |
Female wild-type BALB/c mice (4-5 weeks, 18 ± 2 g, n=8/group, allergic conjunctivitis mouse model established using histamine or an antigen (ovalbumin)) 0.2 mg/mL, 10 µl per eye Intraperitoneal injection (IP), once |
| ADME/Pharmacokinetics |
Absorption, Distribution and Excretion Following topical application of naphazoline hydrochloride solutions to the conjunctiva, local vasoconstriction usually occurs within 10 minutes and may persist for 2-6 hours. Occasionally, enough naphazoline may be absorbed to produce systemic effects. Information on the distribution and elimination of the drug in humans is not available |
| Toxicity/Toxicokinetics |
Interactions Patients being treated with monoamine oxidase (MAO) inhibitors may experience a severe hypertensive reaction if administered with a sympathomimetic drug. Although this reaction has not specifically been reported with naphazoline, the possibility of such an interaction should be considered. Concurrent use of maprotiline or tricyclic antidepressants with naphazoline hydrochloride may potentiate the pressor effects of naphazoline. PENTOBARBITONE APPEARS TO ENHANCE HYPOTENSIVE ACTION OF ALPHA-SYMPATHOMIMETIC DRUGS, INCL NAPHAZOLINE HYDROCHLORIDE. Non-Human Toxicity Values LD50 Rat sc 385 mg/kg |
| References |
[1]. Treatment with olopatadine and naphazoline hydrochloride reduces allergic conjunctivitis in mice through alterations in inflammation, NGF and VEGF. Mol Med Rep. 2016 Apr;13(4):3319-25. [2]. Central and peripheral adrenergic mechanisms regulating gastric secretion in the rat. J Pharmacol Exp Ther. 1977 Oct;203(1):125-31. |
| Additional Infomation |
Naphazoline is an organic molecular entity. Naphazoline Hydrochloride is the hydrochloride salt form of naphazoline, an imidazole derivative and a direct-acting sympathomimetic amine with vasoconstrictive properties. Upon ocular administration, naphazoline hydrochloride exerts its effect by acting on alpha-adrenergic receptors in the arterioles of the conjunctiva to produce vasoconstriction, resulting in decreased conjunctival congestion and diminished itching, irritation and redness. An adrenergic vasoconstrictor agent used as a decongestant. See also: Naphazoline (has active moiety); Naphazoline Hydrochloride; Pheniramine Maleate (component of); Antazoline phosphate; naphazoline hydrochloride (component of) ... View More ... Mechanism of Action Naphazoline constricts the vascular system of the conjunctiva. It is presumed that this effect is due to direct stimulation action of the drug upon the alpha-adrenergic receptors in the arterioles of the conjunctiva, resulting in decreased conjunctival congestion. The mechanism of action of naphazoline has not been conclusively determined. Most pharmacologists believe that the drug directly stimulates alpha-adrenergic receptors of the sympathetic nervous system and exerts little or no effect on beta-adrenergic receptors. Following topical application of naphazoline to the conjunctiva, small arterioles are constricted and conjunctival congestion is temporarily relieved, but reactive hyperemia may occur. The drug also may produce mydriasis when applied to the conjunctiva, but this effect is usually minimal with the concentrations used as ocular decongestants. Therapeutic Uses Adrenergic alpha-Agonists; Nasal Decongestants Naphazoline is applied topically to the conjunctiva to temporarily relieve congestion, itching, and minor irritation. Ocular decongestants are ineffective in the treatment of delayed hypersensitivity reactions such as contact dermatoconjunctivitis. The vasoconstrictor effects of naphazoline may be used during some ocular diagnostic procedures, but some clinicians prefer phenylephrine to naphazoline for this use. Ophthalmic solutions containing naphazoline in combination with antihistamines such as antazoline phosphate or pheniramine maleate and/or astringents such as zinc sulfate are commercially available. In the concentrations usually employed, zinc sulfate is a relatively ineffective antiseptic and may promote vasodilation. Adrenergic (vasoconstrictor); decongestant. Naphazoline hydrochloride (0.1%), an imidazole derivative with preferential alpha-2 activity, /was instilled/ in 17 eyes of 12 patients with myopathic ptosis due to involvement of the levator palpebrae superioris, in the attempt to selectively stimulate Muller's smooth muscle. Naphazoline significantly widened the palpebral fissure with little change in pupillary diameter and no significant change in ocular pressure, visual acuity and near point determination. However, a reduction of the effect, probably due to tachyphylaxis, was noticed when using naphazoline regularly several times a day for few weeks. In conclusion naphazoline has powerful cosmetical and functional effects in mild to moderate myopathic ptosis above all if taken occasionally. Drug Warnings Ophthalmic use of naphazoline may occasionally cause systemic sympathomimetic effects such as headache, hypertension, cardiac irregularities, nervousness, nausea, dizziness, weakness, and sweating. Use of naphazoline in the eye may cause blurred vision, mild transient stinging and/or irritation, mydriasis, and increased or decreased intraocular pressure. Conjunctival application of naphazoline, especially when high concentrations are used in geriatric patients, may liberate pigment granules, presumably from the iris. Rebound congestion, characterized by reactive hyperemia frequently occurs with prolonged use and may result in overuse of the drug. Prolonged use of the drug should be avoided for these reasons. The incidence of serious adverse effects is low in patients receiving therapeutic dosages of ophthalmic solutions of naphazoline hydrochloride. When naphazoline hydrochloride is used in combination preparations, the cautions applicable to each ingredient in the formulation must be observed. Excessive dosage and/or prolonged or too frequent use may irritate the conjunctiva and, especially in children, cause adverse systemic effects. Naphazoline hydrochloride ophthalmic solution should be used with caution in patients with hypertension, cardiovascular abnormalities, diabetes mellitus, hyperthyroidism, infection, or injury. For more Drug Warnings (Complete) data for NAPHAZOLINE HYDROCHLORIDE (14 total), please visit the HSDB record page. |
Solubility Data
| Solubility (In Vitro) |
|
|||
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.5 mg/mL (10.13 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 2.5 mg/mL (10.13 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 3: ≥ 2.5 mg/mL (10.13 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), suspension solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. Solubility in Formulation 4: 75 mg/mL (303.96 mM) in PBS (add these co-solvents sequentially from left to right, and one by one), clear solution; with ultrasonication. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 4.0528 mL | 20.2642 mL | 40.5285 mL | |
| 5 mM | 0.8106 mL | 4.0528 mL | 8.1057 mL | |
| 10 mM | 0.4053 mL | 2.0264 mL | 4.0528 mL |