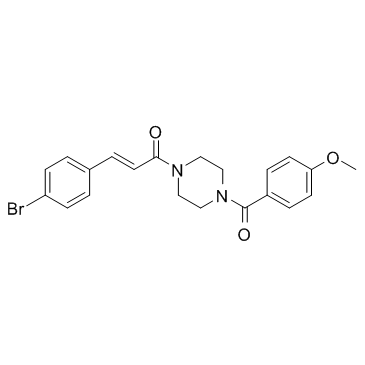
Physicochemical Properties
| Molecular Formula | C21H21BRN2O3 |
| Molecular Weight | 429.314 |
| Exact Mass | 428.074 |
| Elemental Analysis | C, 58.75; H, 4.93; Br, 18.61; N, 6.53; O, 11.18 |
| CAS # | 1599432-08-2 |
| PubChem CID | 36295259 |
| Appearance | White to off-white solid powder |
| LogP | 3.331 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 4 |
| Heavy Atom Count | 27 |
| Complexity | 530 |
| Defined Atom Stereocenter Count | 0 |
| SMILES | BrC1C([H])=C([H])C(=C([H])C=1[H])/C(/[H])=C(\[H])/C(N1C([H])([H])C([H])([H])N(C(C2C([H])=C([H])C(=C([H])C=2[H])OC([H])([H])[H])=O)C([H])([H])C1([H])[H])=O |
| InChi Key | OFHXXBRBGWUOHR-NYYWCZLTSA-N |
| InChi Code | InChI=1S/C21H21BrN2O3/c1-27-19-9-5-17(6-10-19)21(26)24-14-12-23(13-15-24)20(25)11-4-16-2-7-18(22)8-3-16/h2-11H,12-15H2,1H3/b11-4+ |
| Chemical Name | (E)-3-(4-bromophenyl)-1-[4-(4-methoxybenzoyl)piperazin-1-yl]prop-2-en-1-one |
| Synonyms | NIBR189; NIBR 189; NIBR-189 |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | EBI2 ( IC50 = 11 nM ); EBI2 ( IC50 = 16 nM ) |
| ln Vitro | NIBR189, an EBI2 antagonist, inhibits migration with an IC50 of 0.3 nM in U937 cells expressing functional EBI2 levels.[1] |
| ln Vivo | NIBR189 possesses pharmacokinetic qualities that should enable its application in both in vitro and in vivo research. It is a strong and selective EBI2 antagonist.[1] |
| Cell Assay | U937 cells were cultured in lipid-depleted media for the entire night before the migration assay. Using HTS Transwell-96 plates with 5.0 μm pore polycarbonate membranes, chemotaxis was carried out. Let's recap: 240 μL of compound solution in media containing 1% BSA instead of FCS was placed in lower chambers, and 0.75×105 cells in 75 μL media were added to the upper chamber once the filters were inserted. Using flow cytometry, the cells in the lower chamber were examined after three hours at 37°C. |
| Animal Protocol |
C57BL/6 male mice 1 mg/kg (i.v.); 3 mg/kg (p.o.) i.v., p.o. |
| References |
[1]. Identification and characterization of small molecule modulators of the Epstein-Barr virus-induced gene 2 (EBI2) receptor. J Med Chem. 2014 Apr 24;57(8):3358-68. |
Solubility Data
| Solubility (In Vitro) |
DMSO: ~21~50 mg/mL (~48.9~116.5 mM) Ethanol: ~4 mg/mL |
| Solubility (In Vivo) |
Solubility in Formulation 1: 2.5 mg/mL (5.82 mM) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), suspension solution; with sonication. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 2: ≥ 2.5 mg/mL (5.82 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 3: (saturation unknown) in 5%DMSO + 40%PEG300 + 5%Tween 80+ 50%ddH2O:1.05mg/ml (2.45mM) (add these co-solvents sequentially from left to right, and one by one), Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.3293 mL | 11.6466 mL | 23.2932 mL | |
| 5 mM | 0.4659 mL | 2.3293 mL | 4.6586 mL | |
| 10 mM | 0.2329 mL | 1.1647 mL | 2.3293 mL |