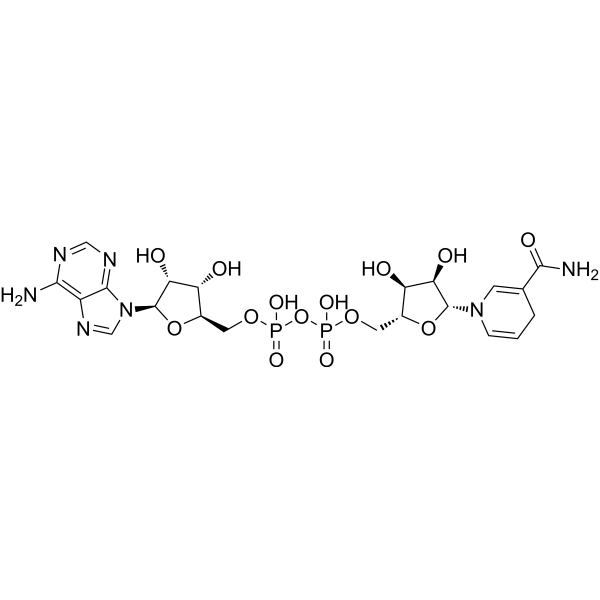
Physicochemical Properties
| Molecular Formula | C21H29N7O14P2 |
| Molecular Weight | 665.44 |
| Exact Mass | 665.124 |
| Elemental Analysis | C, 37.90; H, 4.39; N, 14.73; O, 33.66; P, 9.31 |
| CAS # | 58-68-4 |
| Related CAS # | 53-84-9 (free acid); 58-68-4 (reduced); 20111-18-6 (sodium) |
| PubChem CID | 439153 |
| Appearance | Typically exists as solid at room temperature |
| Density | 2.2±0.1 g/cm3 |
| Boiling Point | 1081.8±75.0 °C at 760 mmHg |
| Melting Point |
140.0-142.0 °C 140.0 - 142.0 °C |
| Flash Point | 608.0±37.1 °C |
| Vapour Pressure | 0.0±0.3 mmHg at 25°C |
| Index of Refraction | 1.845 |
| LogP | -4.35 |
| Hydrogen Bond Donor Count | 8 |
| Hydrogen Bond Acceptor Count | 19 |
| Rotatable Bond Count | 11 |
| Heavy Atom Count | 44 |
| Complexity | 1230 |
| Defined Atom Stereocenter Count | 8 |
| SMILES | C1C=CN(C=C1C(=O)N)[C@H]2[C@@H]([C@@H]([C@H](O2)COP(=O)(O)OP(=O)(O)OC[C@@H]3[C@H]([C@H]([C@@H](O3)N4C=NC5=C(N=CN=C54)N)O)O)O)O |
| InChi Key | BOPGDPNILDQYTO-NNYOXOHSSA-N |
| InChi Code | InChI=1S/C21H29N7O14P2/c22-17-12-19(25-7-24-17)28(8-26-12)21-16(32)14(30)11(41-21)6-39-44(36,37)42-43(34,35)38-5-10-13(29)15(31)20(40-10)27-3-1-2-9(4-27)18(23)33/h1,3-4,7-8,10-11,13-16,20-21,29-32H,2,5-6H2,(H2,23,33)(H,34,35)(H,36,37)(H2,22,24,25)/t10-,11-,13-,14-,15-,16-,20-,21-/m1/s1 |
| Chemical Name | [[(2R,3S,4R,5R)-5-(6-aminopurin-9-yl)-3,4-dihydroxyoxolan-2-yl]methoxy-hydroxyphosphoryl] [(2R,3S,4R,5R)-5-(3-carbamoyl-4H-pyridin-1-yl)-3,4-dihydroxyoxolan-2-yl]methyl hydrogen phosphate |
| Synonyms | Adenosine 5'-(trihydrogen diphosphate), P'.fwdarw.5'-ester with 1,4-dihydro-1-.beta.-D-ribofuranosyl-3-pyridinecarboxamide; Adenosine 5'-(trihydrogen diphosphate), P'.fwdarw.5'-ester with 1,4-dihydro-1-beta-D-ribofuranosyl-3-pyridinecarboxamide; NADH; DPNH; 58-68-4; beta-DPNH; beta-NADH; 1,4-Dihydronicotinamide adenine dinucleotide; |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | Endogenous Metabolite |
| ln Vitro | Increasing evidence has indicated that NAD+ and NADH play critical roles not only in energy metabolism, but also in cell death and various cellular functions including regulation of calcium homeostasis and gene expression. It has also been indicated that NAD+ and NADH are mediators of multiple major biological processes including aging. NAD+ and NADH produce the biological effects by regulating numerous NAD+/NADH-dependent enzymes, including dehydrogenases, poly(ADP-ribose) polymerases, Sir2 family proteins (sirtuins), mono(ADP-ribosyl)transferases, and ADP-ribosyl cyclases. Of particular interest, NAD+-dependent generation of ADP-ribose, cyclic ADP-ribose and O-acetyl-ADP-ribose can mediate calcium homeostasis by affecting TRPM2 receptors and ryanodine receptors; and sirtuins and PARPs appear to play key roles in aging, cell death and a variety of cellular functions. It has also been indicated that NADH and NAD+ can be transported across plasma membranes of cells, and that extracellular NAD+ may be a new signaling molecule. Our latest studies have shown that intranasal NAD+ administration can profoundly decrease ischemic brain damage. These new pieces of information have fundamentally changed our understanding about NAD+ and NADH, suggesting novel paradigms about the metabolism and biological activities of NAD+ and NADH. Based on this information, it is tempted to hypothesize that NAD+ and NADH, together with ATP and Ca2+, may be four most fundamental components in life, which can significantly affect nearly all major biological processes. Future studies on NAD+ and NADH may not only elucidate some fundamental mysteries in biology, but also provide novel insights for interfering aging and many disease processes [1]. |
| ADME/Pharmacokinetics |
Absorption, Distribution and Excretion Unclear how much of an administered dose is absorbed. |
| References |
[1]. Ying W. NAD+ and NADH in cellular functions and cell death. Front Biosci. 2006 Sep 1;11:3129-48. |
| Additional Infomation |
NADH is a coenzyme found in all living cells; consists of two nucleotides joined through their 5'-phosphate groups, with one nucleotide containing an adenine base and the other containing nicotinamide. It has a role as a fundamental metabolite and a cofactor. It is a NAD(P)H and a NAD. It is a conjugate acid of a NADH(2-). NADH is the reduced form of NAD+, and NAD+ is the oxidized form of NADH, a coenzyme composed of ribosylnicotinamide 5'-diphosphate coupled to adenosine 5'-phosphate by pyrophosphate linkage. It is found widely in nature and is involved in numerous enzymatic reactions in which it serves as an electron carrier by being alternately oxidized (NAD+) and reduced (NADH). It forms NADP with the addition of a phosphate group to the 2' position of the adenosyl nucleotide through an ester linkage. (Dorland, 27th ed) NADH is a metabolite found in or produced by Escherichia coli (strain K12, MG1655). 1,4-Dihydronicotinamide adenine dinucleotide has been reported in Arabidopsis thaliana, Homo sapiens, and other organisms with data available. A coenzyme composed of ribosylnicotinamide 5'-diphosphate coupled to adenosine 5'-phosphate by pyrophosphate linkage. It is found widely in nature and is involved in numerous enzymatic reactions in which it serves as an electron carrier by being alternately oxidized (NAD+) and reduced (NADH). (Dorland, 27th ed) Drug Indication Some evidence suggests that NADH might be useful in treating Parkinson's disease, chronic fatigue syndrome, Alzheimer's disease and cardiovascular disease. Mechanism of Action NADH is synthesized by the body and thus is not an essential nutrient. It does require the essential nutrient nicotinamide for its synthesis, and its role in energy production is certainly an essential one. In addition to its role in the mitochondrial electron transport chain, NADH is produced in the cytosol. The mitochondrial membrane is impermeable to NADH, and this permeability barrier effectively separates the cytoplasmic from the mitochondrial NADH pools. However, cytoplasmic NADH can be used for biologic energy production. This occurs when the malate-aspartate shuttle introduces reducing equivalents from NADH in the cytosol to the electron transport chain of the mitochondria. This shuttle mainly occurs in the liver and heart. Pharmacodynamics A coenzyme composed of ribosylnicotinamide 5'-diphosphate coupled to adenosine 5'-phosphate by pyrophosphate linkage. It is found widely in nature and is involved in numerous enzymatic reactions in which it serves as an electron carrier by being alternately oxidized (NAD+) and reduced (NADH). The action of supplemental NADH is unclear. Oral NADH supplementation has been used to combat simple fatigue as well as such mysterious and energy-sapping disorders as chronic fatigue syndrome and fibromyalgia. Researchers are also studying the value of NADH supplements for improving mental function in people with Alzheimer's disease, and minimizing physical disability and relieving depression in people with Parkinson's disease. Some healthy individuals also take NADH supplements orally to improve concentration and memory capacity, as well as to increase athletic endurance. However, to date there have been no published studies to indicate that using NADH is in any way effective or safe for these purposes. |
Solubility Data
| Solubility (In Vitro) | May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples |
| Solubility (In Vivo) |
Note: Listed below are some common formulations that may be used to formulate products with low water solubility (e.g. < 1 mg/mL), you may test these formulations using a minute amount of products to avoid loss of samples. Injection Formulations (e.g. IP/IV/IM/SC) Injection Formulation 1: DMSO : Tween 80: Saline = 10 : 5 : 85 (i.e. 100 μL DMSO stock solution → 50 μL Tween 80 → 850 μL Saline) *Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH ₂ O to obtain a clear solution. Injection Formulation 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL DMSO → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Injection Formulation 3: DMSO : Corn oil = 10 : 90 (i.e. 100 μL DMSO → 900 μL Corn oil) Example: Take the Injection Formulation 3 (DMSO : Corn oil = 10 : 90) as an example, if 1 mL of 2.5 mg/mL working solution is to be prepared, you can take 100 μL 25 mg/mL DMSO stock solution and add to 900 μL corn oil, mix well to obtain a clear or suspension solution (2.5 mg/mL, ready for use in animals). Injection Formulation 4: DMSO : 20% SBE-β-CD in saline = 10 : 90 [i.e. 100 μL DMSO → 900 μL (20% SBE-β-CD in saline)] *Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Injection Formulation 5: 2-Hydroxypropyl-β-cyclodextrin : Saline = 50 : 50 (i.e. 500 μL 2-Hydroxypropyl-β-cyclodextrin → 500 μL Saline) Injection Formulation 6: DMSO : PEG300 : castor oil : Saline = 5 : 10 : 20 : 65 (i.e. 50 μL DMSO → 100 μLPEG300 → 200 μL castor oil → 650 μL Saline) Injection Formulation 7: Ethanol : Cremophor : Saline = 10: 10 : 80 (i.e. 100 μL Ethanol → 100 μL Cremophor → 800 μL Saline) Injection Formulation 8: Dissolve in Cremophor/Ethanol (50 : 50), then diluted by Saline Injection Formulation 9: EtOH : Corn oil = 10 : 90 (i.e. 100 μL EtOH → 900 μL Corn oil) Injection Formulation 10: EtOH : PEG300:Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL EtOH → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Oral Formulations Oral Formulation 1: Suspend in 0.5% CMC Na (carboxymethylcellulose sodium) Oral Formulation 2: Suspend in 0.5% Carboxymethyl cellulose Example: Take the Oral Formulation 1 (Suspend in 0.5% CMC Na) as an example, if 100 mL of 2.5 mg/mL working solution is to be prepared, you can first prepare 0.5% CMC Na solution by measuring 0.5 g CMC Na and dissolve it in 100 mL ddH2O to obtain a clear solution; then add 250 mg of the product to 100 mL 0.5% CMC Na solution, to make the suspension solution (2.5 mg/mL, ready for use in animals). Oral Formulation 3: Dissolved in PEG400 Oral Formulation 4: Suspend in 0.2% Carboxymethyl cellulose Oral Formulation 5: Dissolve in 0.25% Tween 80 and 0.5% Carboxymethyl cellulose Oral Formulation 6: Mixing with food powders Note: Please be aware that the above formulations are for reference only. InvivoChem strongly recommends customers to read literature methods/protocols carefully before determining which formulation you should use for in vivo studies, as different compounds have different solubility properties and have to be formulated differently. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.5028 mL | 7.5138 mL | 15.0277 mL | |
| 5 mM | 0.3006 mL | 1.5028 mL | 3.0055 mL | |
| 10 mM | 0.1503 mL | 0.7514 mL | 1.5028 mL |