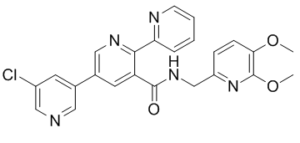
MK-1064 (MK1064) is a novel, potent, selective and orally bioavailable antagonist of Orexin OX2 Receptor (OX2R), with the potential to be used for the treatment of insomnia. Orexin receptors (OX1R, OX2R) are the primary mediators of arousal promotion, and they are used by orexin neuropeptides to control sleep/wake cycles. An antagonist of OX2R singlet is MK-1064. MK-1064 has been shown in preclinical settings to enhance sleep in rats by increasing both REM and NREM sleep at OX2R occupancies higher than those seen with dual orexin receptor antagonists. Like dual antagonists, MK-1064 helps dogs sleep longer in both NREM and REM phases without making them cataplexy. MK-1064 demonstrated dose-dependent increases in subjective somnolence (via Karolinska Sleepiness Scale and Visual Analogue Scale measures) and sleep (via polysomnography), including increased REM and NREM sleep.
Physicochemical Properties
| Molecular Formula | C24H20CLN5O3 | |
| Molecular Weight | 461.91 | |
| Exact Mass | 461.125 | |
| Elemental Analysis | C, 62.41; H, 4.36; Cl, 7.67; N, 15.16; O, 10.39 | |
| CAS # | 1207253-08-4 | |
| Related CAS # |
|
|
| PubChem CID | 44633765 | |
| Appearance | White to off-white solid powder | |
| Density | 1.3±0.1 g/cm3 | |
| Boiling Point | 662.4±55.0 °C at 760 mmHg | |
| Flash Point | 354.4±31.5 °C | |
| Vapour Pressure | 0.0±2.0 mmHg at 25°C | |
| Index of Refraction | 1.619 | |
| LogP | 3.04 | |
| Hydrogen Bond Donor Count | 1 | |
| Hydrogen Bond Acceptor Count | 7 | |
| Rotatable Bond Count | 7 | |
| Heavy Atom Count | 33 | |
| Complexity | 629 | |
| Defined Atom Stereocenter Count | 0 | |
| SMILES | ClC1=C([H])N=C([H])C(=C1[H])C1C([H])=NC(C2=C([H])C([H])=C([H])C([H])=N2)=C(C(N([H])C([H])([H])C2C([H])=C([H])C(=C(N=2)OC([H])([H])[H])OC([H])([H])[H])=O)C=1[H] |
|
| InChi Key | CKTWQGHVNRYNCM-UHFFFAOYSA-N | |
| InChi Code | InChI=1S/C24H20ClN5O3/c1-32-21-7-6-18(30-24(21)33-2)14-29-23(31)19-10-16(15-9-17(25)13-26-11-15)12-28-22(19)20-5-3-4-8-27-20/h3-13H,14H2,1-2H3,(H,29,31) | |
| Chemical Name | 5-(5-chloropyridin-3-yl)-N-[(5,6-dimethoxypyridin-2-yl)methyl]-2-pyridin-2-ylpyridine-3-carboxamide | |
| Synonyms |
|
|
| HS Tariff Code | 2934.99.9001 | |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | OX1 ( IC50 = 1789 nM ); OX2 ( IC50 = 18 nM ); OX1 ( Ki = 1584 nM ); OX2 ( Ki = 0.5 nM ) | |
| ln Vitro |
|
|
| ln Vivo |
|
|
| Animal Protocol |
|
|
| References |
[1]. Discovery of 5''-chloro-N-[(5,6-dimethoxypyridin-2-yl)methyl]-2,2':5',3''-terpyridine-3'-carboxamide (MK-1064): a selective orexin 2 receptor antagonist (2-SORA) for the treatment of insomnia. ChemMedChem. 2014 Feb;9(2):311-22. [2]. Orexin 2 Receptor Antagonism is Sufficient to Promote NREM and REM Sleep from Mouse to Man. Sci Rep. 2016 Jun 3;6:27147. [3]. Orexin 2 receptor regulation of the hypothalamic-pituitary-adrenal (HPA) response to acute and repeated stress. Neuroscience. 2017 Apr 21;348:313-323. |
|
| Additional Infomation |
MK-1064 is under investigation in clinical trial NCT02549014 (A Single Dose Study of the Safety, Pharmacokinetics and Pharmacodynamics of MK-1064 (MK-1064-001)). The field of small-molecule orexin antagonist research has evolved rapidly in the last 15 years from the discovery of the orexin peptides to clinical proof-of-concept for the treatment of insomnia. Clinical programs have focused on the development of antagonists that reversibly block the action of endogenous peptides at both the orexin 1 and orexin 2 receptors (OX1R and OX2R), termed dual orexin receptor antagonists (DORAs), affording late-stage development candidates including Merck’s suvorexant (new drug application filed 2012). Full characterization of the pharmacology associated with antagonism of either OX1R or OX2R alone has been hampered by the dearth of suitable subtype-selective, orally bioavailable ligands. Herein, we report the development of a selective orexin 2 antagonist (2-SORA) series to afford a potent, orally bioavailable 2-SORA ligand. Several challenging medicinal chemistry issues were identified and overcome during the development of these 2,5-disubstituted nicotinamides, including reversible CYP inhibition, physiochemical properties, P-glycoprotein efflux and bioactivation. This article highlights structural modifications the team utilized to drive compound design, as well as in vivo characterization of our 2-SORA clinical candidate, 5′′-chloro-N-[(5,6-dimethoxypyridin-2-yl)methyl]-2,2′:5′,3′′-terpyridine-3′-carboxamide (MK-1064), in mouse, rat, dog, and rhesus sleep models.[1] Orexin neuropeptides regulate sleep/wake through orexin receptors (OX1R, OX2R); OX2R is the predominant mediator of arousal promotion. The potential for single OX2R antagonism to effectively promote sleep has yet to be demonstrated in humans. MK-1064 is an OX2R-single antagonist. Preclinically, MK-1064 promotes sleep and increases both rapid eye movement (REM) and non-REM (NREM) sleep in rats at OX2R occupancies higher than the range observed for dual orexin receptor antagonists. Similar to dual antagonists, MK-1064 increases NREM and REM sleep in dogs without inducing cataplexy. Two Phase I studies in healthy human subjects evaluated safety, tolerability, pharmacokinetics and sleep-promoting effects of MK-1064, and demonstrated dose-dependent increases in subjective somnolence (via Karolinska Sleepiness Scale and Visual Analogue Scale measures) and sleep (via polysomnography), including increased REM and NREM sleep. Thus, selective OX2R antagonism is sufficient to promote REM and NREM sleep across species, similarly to that seen with dual orexin receptor antagonism.[2] Orexins are hypothalamic neuropeptides that have a documented role in mediating the acute stress response. However, their role in habituation to repeated stress, and the role of orexin receptors (OX1R and OX2R) in the stress response, has yet to be defined. Orexin neuronal activation and levels in the cerebrospinal fluid (CSF) were found to be stimulated with acute restraint, but were significantly reduced by day five of repeated restraint. As certain disease states such as panic disorder are associated with increased central orexin levels and failure to habituate to repeated stress, the effect of activating orexin signaling via Designer Receptors Exclusively Activated by Designer Drugs (DREADDs) on the hypothalamic-pituitary-adrenal (HPA) response was evaluated after repeated restraint. While vehicle-treated rats displayed habituation of Adrenocorticotropic Hormone (ACTH) from day 1 to day 5 of restraint, stimulating orexins did not further increase ACTH beyond vehicle levels for either acute or repeated restraint. We delineated the roles of orexin receptors in acute and repeated stress using a selective OX2R antagonist (MK-1064). Pretreatment with MK-1064 reduced day 1 ACTH levels, but did not allow further habituation on day 5 compared with vehicle-treated rats, indicating that endogenous OX2R activity plays a role in acute stress, but not in habituation to repeated stress. However, in restrained rats with further stimulated orexins by DREADDs, MK-1064 decreased ACTH levels on day 5. Collectively, these results indicate that the OX2R plays a role in acute stress, and can prevent habituation to repeated stress under conditions of high orexin release.[3] |
Solubility Data
| Solubility (In Vitro) |
|
|||
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.5 mg/mL (5.41 mM) (saturation unknown) in 10% DMSO + 40% PEG300 +5% Tween-80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 + to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.1649 mL | 10.8246 mL | 21.6492 mL | |
| 5 mM | 0.4330 mL | 2.1649 mL | 4.3298 mL | |
| 10 mM | 0.2165 mL | 1.0825 mL | 2.1649 mL |