Laropiprant (aslo known as MK-0524; Cordaptive) is a novel, potent, selective DP [prostaglandin D2 (PGD2) receptor (DP)] receptor antagonist with Ki values of 0.57 nM and 2.95 nM for DP receptor and TP Receptor, respectively. Laropiprant, which was formerly prescribed in conjunction with niacin to lower blood cholesterol (LDL and VLDL), is no longer in production because of an increase in adverse effects that outweigh any cardiovascular benefits. The U.S. Food and Drug Administration (FDA) sent a "not approved" letter for Cordaptive on April 28, 2008. On July 3, 2008, the European Medicines Agency (EMA) approved Tredaptive. On January 11, 2013, Merck & Co. Inc. declared that they were discontinuing the medication globally due to suggestions from European regulators.
Physicochemical Properties
| Molecular Formula | C21H19CLFNO4S |
| Molecular Weight | 435.89600 |
| Exact Mass | 435.07 |
| Elemental Analysis | C, 57.87; H, 4.39; Cl, 8.13; F, 4.36; N, 3.21; O, 14.68; S, 7.35 |
| CAS # | 571170-77-9 |
| Related CAS # | Laropiprant sodium; 572874-50-1 |
| PubChem CID | 9867642 |
| Appearance | White to off-white solid powder |
| Density | 1.5±0.1 g/cm3 |
| Boiling Point | 710.0±60.0 °C at 760 mmHg |
| Melting Point | 175∶ºC |
| Flash Point | 383.2±32.9 °C |
| Vapour Pressure | 0.0±2.4 mmHg at 25°C |
| Index of Refraction | 1.664 |
| LogP | 3.82 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 5 |
| Heavy Atom Count | 29 |
| Complexity | 721 |
| Defined Atom Stereocenter Count | 1 |
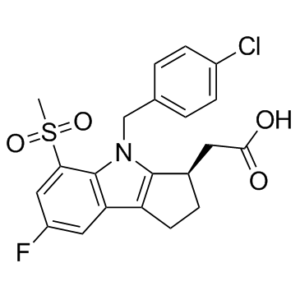
| SMILES | O=S(C1=CC(F)=CC2=C1N(C3=C2CC[C@@H]3CC(O)=O)CC4=CC=C(Cl)C=C4)(C)=O |
| InChi Key | NXFFJDQHYLNEJK-CYBMUJFWSA-N |
| InChi Code | InChI=1S/C21H19ClFNO4S/c1-29(27,28)18-10-15(23)9-17-16-7-4-13(8-19(25)26)20(16)24(21(17)18)11-12-2-5-14(22)6-3-12/h2-3,5-6,9-10,13H,4,7-8,11H2,1H3,(H,25,26)/t13-/m1/s1 |
| Chemical Name | 2-[(3R)-4-[(4-chlorophenyl)methyl]-7-fluoro-5-methylsulfonyl-2,3-dihydro-1H-cyclopenta[b]indol-3-yl]acetic acid |
| Synonyms | MK-0524; Laropiprant; MK0524; MK 0524; Trade name: Cordaptive; Tredaptive |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | DP ( Ki = 0.57 nM ); TP Receptor ( Ki = 2.95 nM ) | |
| ln Vitro |
|
|
| ln Vivo | Laropiprant (100-100 mg/kg; intravenous and oral; male) Sprague-Dawley rats) show positive pharmacokinetic characteristics. [3]. An analysis of pharmacokinetics in male Sprague-Dawley rats [3] The dosage by route (mg/kg) AUC0-∞ (μM·hr) Clp (mL/min/kg) Vdss (L/kg) T1/2 (hour) PO 5 96.0 2.1 0.9 7.6 PO 1 22.7 1.9 0.7 7.4 The dosage by route (mg/kg) AUC0-∞ (μM·hr) Cmax (micrometer) Tmax in hours F(%) IV 5 15.6 1.2 52.6 / | |
| Cell Assay | Vena8Fluoro+ Biochips are coated with collagen (200 µg/mL) at 4°C for an entire night. After that, they are blocked for 30 minutes at room temperature using bovine serum albumin (10 µg/mL), and then they are cleaned. After being collected in sodium citrate, 1 µM of 3, 3-dihexyloxacarbocyanine iodide is incubated for 10 minutes in the dark with whole blood. Ten minutes prior to the onset of perfusion, PGD2 (30 nM), BW245c (3 nM), and the DP antagonist BWA868c or Laropiprant (1 µM) are added. In an additional series of tests, whole blood is exposed to 30 minutes of treatment with either niacin (3 mM), acetylsalicylic acid (1 mM), or laropiprant (1 µM and 10 µM). A final concentration of 1 mM of CaCl2 is added two minutes prior to the perfusion over the chip coated in collagen. The shear rate at which perfusion occurs is 30 dynes cm^2. The formation of thrombus is noted. DucoCell analysis software is used for computerized image analysis, where the area covered by the thrombus is computed. Percentage of the area covered in a control sample is used to express data[2]. | |
| References |
[1]. Inverse agonist and pharmacochaperone properties of MK-0524 on the prostanoid DP1 receptor. PLoS One. 2013 Jun 10;8(6):e65767. [2]. Discovery of a potent and selective prostaglandin D2 receptor antagonist, [(3R)-4-(4-chloro-benzyl)-7-fluoro-5-(methylsulfonyl)-1,2,3,4-tetrahydrocyclopenta[b]indol-3-yl]-acetic acid (MK-0524). J Med Chem. 2007 Feb 22;50(4):794-806. [3]. The pharmacokinetics and disposition of MK-0524, a Prosglandin D2 Receptor 1 antagonist, in rats, dogs and monkeys. Xenobiotica. 2007 May;37(5):514-33. |
|
| Additional Infomation |
Laropiprant is an indolyl carboxylic acid. Laropiprant is an ingredient in the EMA-withdrawn product Pelzont. Laropiprant is a prostaglandin D2 receptor (DP1) antagonist with niacin-induced vasodilation inhibiting activity. Laropiprant binds to and inhibits the activity of DP1, a G-protein coupled receptor. Via competing with prostaglandin D2 (PG D2) for binding to DP1, this agent prevents PG D2-induced vasodilation and increased blood flow. As niacin induces the synthesis of PG D2, predominantly in the skin, administration of laropiprant may prevent niacin-induced vasodilation in the skin and facial flushing. |
Solubility Data
| Solubility (In Vitro) |
DMSO: ~87 mg/mL (199.6 mM) Ethanol: ~87 mg/mL |
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.5 mg/mL (5.74 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 2.5 mg/mL (5.74 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.2941 mL | 11.4705 mL | 22.9410 mL | |
| 5 mM | 0.4588 mL | 2.2941 mL | 4.5882 mL | |
| 10 mM | 0.2294 mL | 1.1471 mL | 2.2941 mL |