Description: Hesperadin is a novel, potent and ATP-competitive inhibitor of human aurora B kinase with potential antitumor activity. It inhibits Aurora B with IC50 of 250 nM in a cell-free assay. It markedly reduces the activity of AMPK, Lck, MKK1, MAPKAP-K1, CHK1 and PHK while it does not inhibit MKK1 activity in vivo. Hersperadin shows potent in vitro antiproliferative activity and high in vivo antitumor efficacy.
Physicochemical Properties
| Molecular Formula | C29H32N4O3S |
| Molecular Weight | 516.65 |
| Exact Mass | 516.219 |
| CAS # | 422513-13-1 |
| Related CAS # | Hesperadin hydrochloride |
| PubChem CID | 135421442 |
| Appearance | Light yellow to yellow solid powder |
| Density | 1.3±0.1 g/cm3 |
| Index of Refraction | 1.675 |
| LogP | 3.13 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 8 |
| Heavy Atom Count | 37 |
| Complexity | 855 |
| Defined Atom Stereocenter Count | 0 |
| InChi Key | GLDSKRNGVVYJAB-DQSJHHFOSA-N |
| InChi Code | InChI=1S/C29H32N4O3S/c1-2-37(35,36)32-24-15-16-26-25(19-24)27(29(34)31-26)28(22-9-5-3-6-10-22)30-23-13-11-21(12-14-23)20-33-17-7-4-8-18-33/h3,5-6,9-16,19,30,32H,2,4,7-8,17-18,20H2,1H3,(H,31,34)/b28-27- |
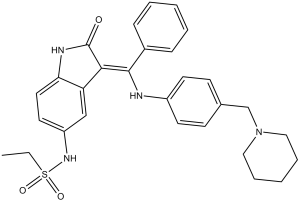
| Chemical Name | (Z)-N-(2-oxo-3-(phenyl((4-(piperidin-1-ylmethyl)phenyl)amino)methylene)indolin-5-yl)ethanesulfonamide |
| Synonyms | Hesperadine; Hesperadin; Hesperadine; |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| ln Vitro | Hesperadin (10-100 nM) suppresses Aurora kinase-1 (TbAUK1)-mediated phosphorylation of trypanosome histone H3 (TbH3) in a dose-dependent manner, with an IC50 of 40 nM [1]. Hesperadin (0.01-10 μM; 24 or 48 hours) reduces the growth of bloodstream (BF) and procyclic (PF) cultures [1]. Hesperadin (100-200 nM; 24-72 hours) affects cell shape and hinders cell cycle progression, similar to RNAi suppression of TbAUK1 [1]. |
| ln Vivo | Hesperadin (20 mg/kg/d; IV) works in concert with temozolomide (TMZ) to extend the survival of xenograft mice[2]. |
| Cell Assay |
Cell Viability Assay[1] Cell Types: M110 cells Tested Concentrations: 0.01, 0.1, 1, 10 μM Incubation Duration: 24 hrs (hours) or 48 hrs (hours) Experimental Results: Inhibiting growth of BF cultures with IC50 of 50 nM, while the inhibition of PF growth required approximately 11-fold more Hesperadin, with IC50 of 550 nM. Cell Cycle Analysis[1] Cell Types: M110 cells Tested Concentrations: 100, 200 nM Incubation Duration: 24, 48, 72 hrs (hours) Experimental Results: Had a strong effect on cell growth and mitotic progression at 100-200 nM. |
| Animal Protocol |
Animal/Disease Models: 6weeks old female nude mice injected GBM cells[2] Doses: 20 mg /kg/d Route of Administration: Iv injection Experimental Results: Increased the survival of xenograft mice models. |
| References |
[1]. The cell cycle as a therapeutic target against Trypanosoma brucei: Hesperadin inhibits Aurora kinase-1 and blocks mitotic progression in bloodstream forms. Mol Microbiol. 2009 Apr; 72(2): 442-58. [2]. Targeting Aurora kinase B attenuates chemoresistance in glioblastoma via a synergistic manner with temozolomide. Pathol Res Pract. 2019 Nov; 215(11): 152617. [3]. Chemical Genomics Approach Leads to the Identification of Hesperadin, an Aurora B Kinase Inhibitor, as a Broad-Spectrum Influenza Antiviral. Int J Mol Sci. 2017 Sep 8;18(9):1929. |
| Additional Infomation | Hesperadin is an oxindole that is indolin-2-one which is substituted at position 5 by an (ethylsulfonyl)nitrilo group and at position 2 by a methylidene group, which is itself substituted by a phenyl group and a [4-(piperidin-1-ylmethyl)phenyl]amino group. An Aurora B kinase inhibitor, it is used to inhibit chromosome alignment and segregation. It has a role as an Aurora kinase inhibitor. It is a member of oxindoles, a member of piperidines, a sulfonamide, an enamine and a tertiary amino compound. |
Solubility Data
| Solubility (In Vitro) |
|
|||
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.5 mg/mL (4.84 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 2.5 mg/mL (4.84 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. Solubility in Formulation 3: 30% PEG400+0.5% Tween80+5% propylene glycol: 30 mg/mL (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.9355 mL | 9.6777 mL | 19.3555 mL | |
| 5 mM | 0.3871 mL | 1.9355 mL | 3.8711 mL | |
| 10 mM | 0.1936 mL | 0.9678 mL | 1.9355 mL |