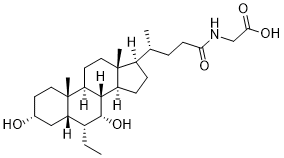
Glyco-Obeticholic acid is an active metabolite of Obeticholic acid formed by a phase II metabolic reaction by conjugating with amino acid glycine. Obeticholic acid (Ocaliva; 6-Ethylchenodeoxycholic acid; 6-ECDCA; INT747) is a farnesoid X receptor (FXR) agonist and semisynthetic bile acid analog (derivative of chenodeoxycholic acid) approved by FDA in 2016 to treat primary biliary cholangitis. Glyco-obeticholic acid may be used as a precursor in the preparation of bile acid analogs as agonists of the farnesoid X receptor (FXR) and TGR5.
Physicochemical Properties
| Molecular Formula | C28H47NO5 |
| Molecular Weight | 477.676489114761 |
| Exact Mass | 477.345 |
| Elemental Analysis | C, 70.40; H, 9.92; N, 2.93; O, 16.75 |
| CAS # | 863239-60-5 |
| Related CAS # | Glyco-obeticholic acid-d5 |
| PubChem CID | 121322333 |
| Appearance | Typically exists as solid at room temperature |
| LogP | 5.1 |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 7 |
| Heavy Atom Count | 34 |
| Complexity | 772 |
| Defined Atom Stereocenter Count | 11 |
| SMILES | CC[C@@H]1[C@@H]2C[C@@H](CC[C@@]2([C@H]3CC[C@]4([C@H]([C@@H]3[C@@H]1O)CC[C@@H]4[C@H](C)CCC(=O)NCC(=O)O)C)C)O |
| InChi Key | MTLPUOZJBFHNSO-FCWTVGIUSA-N |
| InChi Code | InChI=1S/C28H47NO5/c1-5-18-22-14-17(30)10-12-28(22,4)21-11-13-27(3)19(7-8-20(27)25(21)26(18)34)16(2)6-9-23(31)29-15-24(32)33/h16-22,25-26,30,34H,5-15H2,1-4H3,(H,29,31)(H,32,33)/t16-,17-,18-,19-,20+,21+,22+,25+,26-,27-,28-/m1/s1 |
| Chemical Name | 2-[[(4R)-4-[(3R,5S,6R,7R,8S,9S,10S,13R,14S,17R)-6-ethyl-3,7-dihydroxy-10,13-dimethyl-2,3,4,5,6,7,8,9,11,12,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-17-yl]pentanoyl]amino]acetic acid |
| Synonyms | GlycoObeticholic acid; Glyco-obeticholic acid; 863239-60-5; Glyco-oca; 6-Egcdca; O2MZK6V9LQ; UNII-O2MZK6V9LQ; CHEMBL4072790; Glyco Obeticholic acid |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | Active metabolite of Obeticholic acid; phase II reaction product; farnesoid X receptor (FXR) |
| ln Vitro | Microorganisms in the ileum and colon can cleave the sugar obeticholic acid and convert it into the parent drug, which can subsequently be reabsorbed or expelled in the feces. Obeticholic acid interacts with glycine in the liver to generate ethylene glycol obeticholic acid, which is released into the bile [1]. |
| ln Vivo | Obeticholic acid (OCA) is exemplified as a potent drug for treating primary biliary cirrhosis and nonalcoholic fatty liver disease by inhibiting bile acid synthesis. However, it remains unclear whether the effect of OCA is mediated by the function of brown adipose tissue (BAT). In the present study, brown adipogenesis differentiation in vitro and db/db mouse model treated with OCA were used to assess the anti-obesity function by body weight tracking, O2 consumption, food intake, physical activity, glucose tolerance tests. In addition, uncoupling protein 1 (Ucp1) protein expression in brown adipose tissue was measured by western blotting, morphometry of brown adipose tissue was analyzed by hematoxylin and eosin staining. Hepatic steatosis was detected by Oil-Red O staining and serological analysis was performed to assess the effect of OCA on hyperlipidemia. OCA treatment enhanced brown adipocyte cell differentiation and upregulated the expression of the BAT-specific gene Ucp1) in C3H10T1/2 cells in vitro. Consistent with these findings, OCA increased whole-body energy metabolism and glucose homeostasis by enhancing BAT activity in vivo, and ultimately decreased body weight gain in db/db mice. In addition, the results demonstrated that spontaneous hepatic steatosis in db/db mice was ameliorated following OCA treatment. In summary, OCA functioned as a BAT activator to help ameliorate obesity and maintain glucose homeostasis in db/db mice. The present results may provide a novel potential therapeutic approach to activate brown fat in patients with obesity and other metabolic disorders.https://pubmed.ncbi.nlm.nih.gov/34345273/ |
| References | [1]. Markham A, et al. Obeticholic Acid: First Global Approval. Drugs. 2016 Aug;76(12):1221-6. |
| Additional Infomation | We have previously demonstrated that the farnesoid X receptor (FXR) agonist obeticholic acid (OCA) protects the liver via downregulation of hepatic matrix metalloproteinases (MMPs) after ischemia/reperfusion (I/R), which can lead to multiorgan dysfunction. The present study investigated the capacity of OCA to modulate MMPs in distant organs such as the kidney. Male Wistar rats were dosed orally with 10 mg/kg/day of OCA (5 days) and were subjected to 60-min partial hepatic ischemia. After 120-min reperfusion, kidney biopsies (cortex and medulla) and blood samples were collected. Serum creatinine, kidney MMP-2, and MMP-9-dimer, tissue inhibitors of MMPs (TIMP-1, TIMP-2), RECK, TNF-alpha, and IL-6 were monitored. MMP-9-dimer activity in the kidney cortex and medulla increased after hepatic I/R and a reduction was detected in OCA-treated I/R rats. Although not significantly, MMP-2 activity decreased in the cortex of OCA-treated I/R rats. TIMPs and RECK levels showed no significant differences among all groups considered. Serum creatinine increased after I/R and a reduction was detected in OCA-treated I/R rats. The same trend occurred for tissue TNF-alpha and IL-6. Although the underlying mechanisms need further investigation, this is the first study showing, in the kidney, beneficial effects of OCA by reducing TNF-alpha-mediated expression of MMPs after liver I/R.https://pubmed.ncbi.nlm.nih.gov/35631351/ |
Solubility Data
| Solubility (In Vitro) | May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples |
| Solubility (In Vivo) |
Note: Listed below are some common formulations that may be used to formulate products with low water solubility (e.g. < 1 mg/mL), you may test these formulations using a minute amount of products to avoid loss of samples. Injection Formulations (e.g. IP/IV/IM/SC) Injection Formulation 1: DMSO : Tween 80: Saline = 10 : 5 : 85 (i.e. 100 μL DMSO stock solution → 50 μL Tween 80 → 850 μL Saline) *Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH ₂ O to obtain a clear solution. Injection Formulation 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL DMSO → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Injection Formulation 3: DMSO : Corn oil = 10 : 90 (i.e. 100 μL DMSO → 900 μL Corn oil) Example: Take the Injection Formulation 3 (DMSO : Corn oil = 10 : 90) as an example, if 1 mL of 2.5 mg/mL working solution is to be prepared, you can take 100 μL 25 mg/mL DMSO stock solution and add to 900 μL corn oil, mix well to obtain a clear or suspension solution (2.5 mg/mL, ready for use in animals). Injection Formulation 4: DMSO : 20% SBE-β-CD in saline = 10 : 90 [i.e. 100 μL DMSO → 900 μL (20% SBE-β-CD in saline)] *Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Injection Formulation 5: 2-Hydroxypropyl-β-cyclodextrin : Saline = 50 : 50 (i.e. 500 μL 2-Hydroxypropyl-β-cyclodextrin → 500 μL Saline) Injection Formulation 6: DMSO : PEG300 : castor oil : Saline = 5 : 10 : 20 : 65 (i.e. 50 μL DMSO → 100 μLPEG300 → 200 μL castor oil → 650 μL Saline) Injection Formulation 7: Ethanol : Cremophor : Saline = 10: 10 : 80 (i.e. 100 μL Ethanol → 100 μL Cremophor → 800 μL Saline) Injection Formulation 8: Dissolve in Cremophor/Ethanol (50 : 50), then diluted by Saline Injection Formulation 9: EtOH : Corn oil = 10 : 90 (i.e. 100 μL EtOH → 900 μL Corn oil) Injection Formulation 10: EtOH : PEG300:Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL EtOH → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Oral Formulations Oral Formulation 1: Suspend in 0.5% CMC Na (carboxymethylcellulose sodium) Oral Formulation 2: Suspend in 0.5% Carboxymethyl cellulose Example: Take the Oral Formulation 1 (Suspend in 0.5% CMC Na) as an example, if 100 mL of 2.5 mg/mL working solution is to be prepared, you can first prepare 0.5% CMC Na solution by measuring 0.5 g CMC Na and dissolve it in 100 mL ddH2O to obtain a clear solution; then add 250 mg of the product to 100 mL 0.5% CMC Na solution, to make the suspension solution (2.5 mg/mL, ready for use in animals). Oral Formulation 3: Dissolved in PEG400 Oral Formulation 4: Suspend in 0.2% Carboxymethyl cellulose Oral Formulation 5: Dissolve in 0.25% Tween 80 and 0.5% Carboxymethyl cellulose Oral Formulation 6: Mixing with food powders Note: Please be aware that the above formulations are for reference only. InvivoChem strongly recommends customers to read literature methods/protocols carefully before determining which formulation you should use for in vivo studies, as different compounds have different solubility properties and have to be formulated differently. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.0935 mL | 10.4673 mL | 20.9345 mL | |
| 5 mM | 0.4187 mL | 2.0935 mL | 4.1869 mL | |
| 10 mM | 0.2093 mL | 1.0467 mL | 2.0935 mL |