Physicochemical Properties
| Molecular Formula | C15H10O3 |
| Molecular Weight | 238.24 |
| Exact Mass | 238.063 |
| CAS # | 577-85-5 |
| PubChem CID | 11349 |
| Appearance | Light yellow to yellow solid powder |
| Density | 1.367 g/cm3 |
| Boiling Point | 393.7ºC at 760 mmHg |
| Melting Point | 171-172 °C(lit.) |
| Flash Point | 151.5ºC |
| Index of Refraction | 1.679 |
| LogP | 3.165 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 1 |
| Heavy Atom Count | 18 |
| Complexity | 366 |
| Defined Atom Stereocenter Count | 0 |
| InChi Key | HVQAJTFOCKOKIN-UHFFFAOYSA-N |
| InChi Code | InChI=1S/C15H10O3/c16-13-11-8-4-5-9-12(11)18-15(14(13)17)10-6-2-1-3-7-10/h1-9,17H |
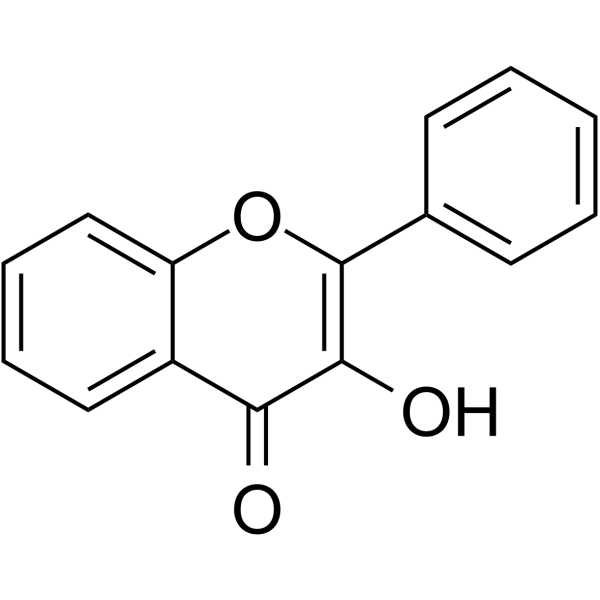
| Chemical Name | 3-hydroxy-2-phenylchromen-4-one |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month Note: This product requires protection from light (avoid light exposure) during transportation and storage. |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| ADME/Pharmacokinetics |
Metabolism / Metabolites It has been reported that flavonoids efficiently protect against peroxynitrite toxicity. Two pharmacophores have been identified in flavonoids, namely the catechol group in ring B and the hydroxyl (OH) group at the 3-position. In this study, this structure-activity relationship was further examined. It was found that catechol (1,2-dihydroxybenzene) is a potent peroxynitrite scavenger, whereas phenol (hydroxybenzene) is not. Of the flavonols tested without a catechol group in ring B, kaempferol (OH groups at positions 3,5,7,4') and galangin (OH groups at positions 3,5,7) are also potent scavengers, whereas apigenin (OH groups at positions 5,7,4') and chrysin (OH groups at positions 5,7) are not. This confirms the importance of the OH group at the 3-position. However, the synthetic flavonol TUM 9761 and 3-hydroxyflavone (OH group only at position 3) are poor scavengers. Based on these results, the structure-activity relationship on the peroxynitrite scavenging activity of flavonols was refined. The catechol in ring B remains important. Also the 3-OH group remains important, but the activity of this pharmacophore is influenced by the substituents at position 5 and at position 7. 3-Hydroxyflavone has known human metabolites that include (2S,3S,4S,5R)-3,4,5-Trihydroxy-6-(4-oxo-2-phenylchromen-3-yl)oxyoxane-2-carboxylic acid. |
| Toxicity/Toxicokinetics |
Interactions ... Male Fischer 344 rats were fed diets supplemented with 0.1% (wt/wt) of /3-hydroxyflavone/ ... and after 2 wk they were treated twice (1 wk apart) with azoxymethane (AOM) (15 mg/kg sc); the dietary treatment continued until sacrifice, 7 wk after the first injection with AOM. ... 3-OH-flavone slightly, although significantly, increased (P < 0.05), the number of ACF per colon (157 +/- 7 and 198 +/- 14 (SE) in control and 3-OH-flavone groups, respectively, n = 10). ... The suppressive effect of flavonoids on the cytotoxicity of linoleic acid hydroperoxide (LOOH) toward rat phenochromocytoma PC12 cells was examined. The extent of cytotoxicity was shown on the basis of % survival determined by the trypan blue exclusion test. On preincubation of cells with either 3-hydroxyflavone, quercetin, or luteolin prior to LOOH exposure, the cytotoxicity was considerably suppressed. In contrast, on coincubation of cells with either eriodictyol, quercetin, kaempherol, luteolin, or 3-hydroxyflavone and LOOH, it was markedly suppressed. Regardless of incubation conditions, quercetin, 3-hydroxyflavone, and luteolin were thus more effective as protective agents against the cytotoxicity than the other flavonoids. These flavonoids further showed a suppressive effect on coincubation rather than on preincubation. .. Non-Human Toxicity Values LD50 Mouse iv 56 mg/kg |
| Additional Infomation |
Flavonol is a monohydroxyflavone that is the 3-hydroxy derivative of flavone. It is a monohydroxyflavone and a member of flavonols. It is a conjugate acid of a flavonol(1-). 3-Hydroxyflavone has been reported in Camellia sinensis, Humulus lupulus, and other organisms with data available. See also: Flavone (annotation moved to). Mechanism of Action Epidermal growth factor (EGF) has been shown to induce proliferation in cells, however, the role of prostaglandin E(2) (PGE(2)) plays in EGF-induced proliferation in still unclear. EGF and PGE(2) showed proliferation responses in epidermoid carcinoma cell A431 by MTT and [(3)H] thymidine incorporation assay. ... The natural product, 3-OH flavone, showed the most-potent inhibitory activity on EGF-induced proliferation among 9 structurally-related compounds, and suppression of EGF receptor phosphorylation, ERK1/2 phosphorylation, and COX-2/PGE(2) production by 3-OH flavone was identified. PGE(2) addition attenuates the inhibitory activity of 3-OH flavone on EGF-induced proliferation by MTT assay and colony formation by soft agar assay. Additionally, 3-OH flavone also showed more-specific inhibition on EGF- than on fetal bovine serum (FBS)-induced proliferation in A431 cells. Results of /the/ present study provide evidence to demonstrate that PGE(2) is an important downstream molecule in EGF-induced proliferation, and 3-OH flavone, which inhibits PGE(2) production by blocking MAPK cascade, might reserve potential for development as an anti-cancer drug. |
Solubility Data
| Solubility (In Vitro) | DMSO: 33.33 mg/mL (139.90 mM) |
| Solubility (In Vivo) |
Solubility in Formulation 1: 2.5 mg/mL (10.49 mM) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), suspension solution; with sonication. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: 2.5 mg/mL (10.49 mM) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), suspension solution; with ultrasonication. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 3: ≥ 2.5 mg/mL (10.49 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 4.1974 mL | 20.9872 mL | 41.9745 mL | |
| 5 mM | 0.8395 mL | 4.1974 mL | 8.3949 mL | |
| 10 mM | 0.4197 mL | 2.0987 mL | 4.1974 mL |