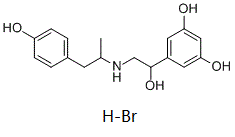
Fenoterol HBr (Th-1165a; Phenoterol hydrobromide), a potent β2 adrenergic agonist designed to open up the airways to the lungs, is classed as sympathomimetic beta agonist and asthma medication.
Physicochemical Properties
| Molecular Formula | C17H22BRNO4 |
| Molecular Weight | 384.26488 |
| Exact Mass | 383.073 |
| Elemental Analysis | C, 53.14; H, 5.77; Br, 20.79; N, 3.65; O, 16.65 |
| CAS # | 1944-12-3 |
| Related CAS # | Fenoterol; 13392-18-2; Fenoterol-d6 hydrobromide; 1286129-04-1 |
| PubChem CID | 5702161 |
| Appearance | White to off-white solid powder |
| Melting Point | 226-228°C |
| Vapour Pressure | 1.19E-13mmHg at 25°C |
| LogP | 3.406 |
| Hydrogen Bond Donor Count | 6 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 6 |
| Heavy Atom Count | 23 |
| Complexity | 310 |
| Defined Atom Stereocenter Count | 0 |
| SMILES | CC(CC1=CC=C(C=C1)O)NCC(C2=CC(=CC(=C2)O)O)O.Br |
| InChi Key | SGZRQMALQBXAIQ-UHFFFAOYSA-N |
| InChi Code | InChI=1S/C17H21NO4.BrH/c1-11(6-12-2-4-14(19)5-3-12)18-10-17(22)13-7-15(20)9-16(21)8-13;/h2-5,7-9,11,17-22H,6,10H2,1H3;1H |
| Chemical Name | 5-[1-hydroxy-2-[1-(4-hydroxyphenyl)propan-2-ylamino]ethyl]benzene-1,3-diol;hydrobromide |
| Synonyms | Fenoterol hydrobromide; Fenoterol HBr; Phenoterol hydrobromide; TH 1165A; TH-1165A; TH1165A; Berotec hydroxyphenylorciprenaline |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month Note: Please store this product in a sealed and protected environment, avoid exposure to moisture. |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | β2 adrenoreceptor |
| ln Vitro |
Fenoterol (1 μM; pre-incubated 30 minutes) treatment significantly downregulates the elevated phosphorylation levels of AMPK and reduces AICAR-induced NF-κB activation, TNF-α release, and AMPK activation[2]. Fenoterol suppresses the production of inflammatory cytokines and AMPK activation caused by lipopolysaccharide (LPS) in THP-1 cells[2]. Fenoterol is also a strong inducer of exosome biogenesis and/or secretion in PC cells[4]. |
| ln Vivo | Fenoterol hydrobromide (0.7 mg/kg; intraperitoneal injection; twice a day; for 3 weeks) suppresses mechanical allodynia during chronic treatment[3]. |
| Cell Assay |
Cell Line: THP-1 cells stimulated with AICAR Concentration: 1 μM Incubation Time: Pre-incubated 30 minutes Result: Significantly downregulated the elevated phosphorylation levels of AMPK. |
| Animal Protocol |
Male C57BL/6J mice (6 weeks old) with neuropathy 0.7 mg/kg Intraperitoneal injection; twice a day; for 3 weeks |
| References |
[1]. Fenoterol: a review of its pharmacological properties and therapeutic efficacy in asthma. Drugs. 1978 Jan;15(1):3-32. [2]. Anti-inflammatory activities of fenoterol through β-arrestin-2 and inhibition of AMPK and NF-κB activation in AICAR-induced THP-1 cells. Biomed Pharmacother. 2016 Dec;84:185-190. [3]. Beta2-adrenoceptor agonists alleviate neuropathic allodynia in mice after chronic treatment. Br J Pharmacol. 2009 Dec;158(7):1683-94. [4]. High-throughput screening identified selective inhibitors of exosome biogenesis and secretion: A drug repurposing strategy for advanced cancer. Sci Rep. 2018 May 25;8(1):8161. |
| Additional Infomation |
Fenoterol hydrobromide is the hydrobromide salt of fenoterol. A beta2-adrenergic agonist, it is used as a bronchodilator in the management of reversible airway obstruction. It has a role as a bronchodilator agent, a beta-adrenergic agonist and a sympathomimetic agent. It contains a fenoterol. Fenoterol Hydrobromide is fenoterol BromideThe hydrobromide salt of fenoterol, a short-acting sympathomimetic agent with bronchodilator activity. Fenoterol stimulates beta-2-adrenergic receptors in the lungs, thereby activating the enzyme adenylate cyclase that catalyzes the conversion of adenosine triphosphate (ATP) to cyclic-3',5'-adenosine monophosphate (cAMP). Increased cAMP concentrations relax bronchial smooth muscle, relieve bronchospasms, and reduce inflammatory cell mediator release, especially from mast cells. A synthetic adrenergic beta-2 agonist that is used as a bronchodilator and tocolytic. |
Solubility Data
| Solubility (In Vitro) |
DMSO: ≥ 100 mg/mL (~260.2 mM) H2O: ~25 mg/mL (~65.1 mM) |
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.5 mg/mL (6.51 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 2.5 mg/mL (6.51 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 3: ≥ 2.5 mg/mL (6.51 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. Solubility in Formulation 4: 60 mg/mL (156.14 mM) in PBS (add these co-solvents sequentially from left to right, and one by one), clear solution; with ultrasonication. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.6024 mL | 13.0120 mL | 26.0240 mL | |
| 5 mM | 0.5205 mL | 2.6024 mL | 5.2048 mL | |
| 10 mM | 0.2602 mL | 1.3012 mL | 2.6024 mL |