Physicochemical Properties
| Molecular Formula | C27H31F3N4O5S |
| Molecular Weight | 580.619055986404 |
| Exact Mass | 580.196 |
| CAS # | 2820151-01-5 |
| Related CAS # | FTI-2153;344900-92-1 |
| PubChem CID | 155981984 |
| Appearance | White to off-white solid powder |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 11 |
| Rotatable Bond Count | 12 |
| Heavy Atom Count | 40 |
| Complexity | 706 |
| Defined Atom Stereocenter Count | 1 |
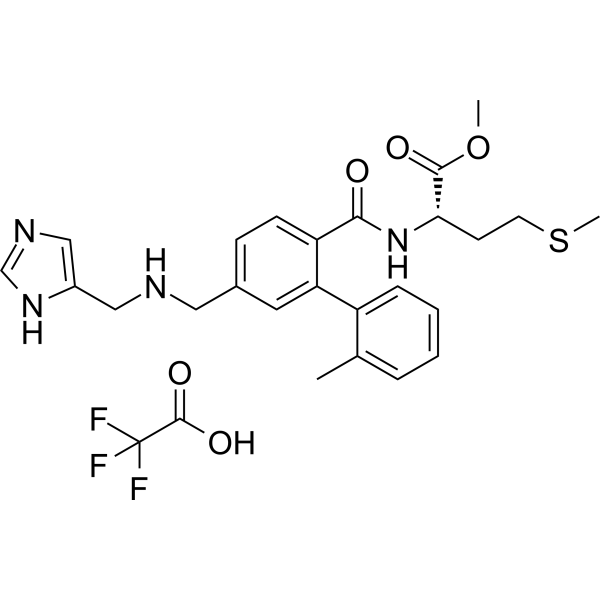
| SMILES | CC1=CC=CC=C1C2=C(C=CC(=C2)CNCC3=CN=CN3)C(=O)N[C@@H](CCSC)C(=O)OC.C(=O)(C(F)(F)F)O |
| InChi Key | LRKNQRCLUQTFRP-BQAIUKQQSA-N |
| InChi Code | InChI=1S/C25H30N4O3S.C2HF3O2/c1-17-6-4-5-7-20(17)22-12-18(13-26-14-19-15-27-16-28-19)8-9-21(22)24(30)29-23(10-11-33-3)25(31)32-2;3-2(4,5)1(6)7/h4-9,12,15-16,23,26H,10-11,13-14H2,1-3H3,(H,27,28)(H,29,30);(H,6,7)/t23-;/m0./s1 |
| Chemical Name | methyl (2S)-2-[[4-[(1H-imidazol-5-ylmethylamino)methyl]-2-(2-methylphenyl)benzoyl]amino]-4-methylsulfanylbutanoate;2,2,2-trifluoroacetic acid |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month Note: Please store this product in a sealed and protected environment (e.g. under nitrogen), avoid exposure to moisture. |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| ln Vitro | In two human lung cancer cell lines, FTI-2153 inhibits bipolar spindle formation during mitosis regardless of transformation and the presence of Ras and p53 mutations[2]. In both transformed and non-transformed cells, the proportion of prometaphase cells with ring-like DNA morphology is increased by FTI-2153[2]. T-24 and Calu-1 cell growth is inhibited by FTI-2153 (15 μM) by 38 and 36%, respectively. Less sensitive, NIH3T3, HFF, and HT-1080 are inhibited by only 8, 8, and 13%, in that order. The growth of A-549 and OVCAR3 cells is suppressed by 25 and 22%, respectively. FTI-2153 inhibits cell growth in both T-24 and Calu-1 cells, but it only affects Calu-1 cells' ability to form bipolar spindles. Only NIH3T3 cells are resistant to FTI-2153 inhibition of bipolar spindle formation, whereas HFF and NIH3T3 cells are resistant to FTI-2153 growth inhibition[2]. |
| Cell Assay |
Cell Viability Assay[2] Cell Types: NIH3T3, HFF, HT1080, T-24, OVCAR3, A-549 and Calu-1 CELLS. Tested Concentrations: 48 h. Incubation Duration: 15 μM. Experimental Results: When A-549 cells were treated with FTI-2153 (15 μM for 48 h) , the proportion of cells at prometaphase increased relative to the other phases of mitosis. FTI-2153 accumulated cells at prometaphase with a rosette-like morphology where chromosomes form a ring surrounding a monoaster of microtubules. In all cells, except for T-24 and NIH3T3, FTI-2153 treatment increased the proportion of mitotic cells in prometaphase and diminished the percentage of cells in telophase/cytokinesis. In HT1080 cells, the percentage of cells in prometaphase and telophase/cytokinesis were 5 and 85% in control cells and 55 and 35% in Treated cells, respectively. Similarly results were also found in HFF cells. Calu-1 and A-549 cells, as described previously, had similarly large changes, whereas OVCAR3 had smaller changes. In contrast, FTI-2153 did not Dramatically aff |
| References |
[1]. Antitumor efficacy of a novel class of non-thiol-containing peptidomimetic inhibitors of farnesyltransferase and geranylgeranyltransferase I: combination therapy with the cytotoxic agents cisplatin, Taxol, and gemcitabine. Cancer Res. 1999 Oct 1;59(19):4919-26. [2]. The farnesyltransferase inhibitor, FTI-2153, inhibits bipolar spindle formation during mitosis independently of transformation and Ras and p53 mutation status. Cell Death Differ. 2002 Jul;9(7):702-9. |
Solubility Data
| Solubility (In Vitro) | DMSO: 90 mg/mL (155.01 mM) |
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.25 mg/mL (3.88 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 22.5 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 2.25 mg/mL (3.88 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 22.5 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 3: ≥ 2.25 mg/mL (3.88 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 22.5 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.7223 mL | 8.6115 mL | 17.2230 mL | |
| 5 mM | 0.3445 mL | 1.7223 mL | 3.4446 mL | |
| 10 mM | 0.1722 mL | 0.8611 mL | 1.7223 mL |