Emedastine is a novel, potent, high affinity, selective, second generation H1-receptor antagonist with pre-clinically well-documented anti-allergic effects. Emedastine's affinity for H1-receptors is 1.3 ±0.1 nM, while its affinity for H2- and H3-receptors is significantly lower, with K1 values of 49,067 ± 11,113 nM and 12,430 ± 1,282 nM, respectively. Since emedastine exhibits pharmacodynamic qualities similar to those of cetirizine, it is a suitable substitute drug with H1-receptor antagonist qualities that is also safe. To support the possible advantages of cetirizine over emedastine following a single dose, more extensive research may be required.
Physicochemical Properties
| Molecular Formula | C25H34N4O9 | |
| Molecular Weight | 534.56 | |
| Exact Mass | 534.233 | |
| Elemental Analysis | C, 56.17; H, 6.41; N, 10.48; O, 26.94 | |
| CAS # | 87233-62-3 | |
| Related CAS # | Emedastine; 87233-61-2; 690625-90-2 (monofumarate) | |
| PubChem CID | 5282485 | |
| Appearance | Solid powder | |
| Boiling Point | 446.6ºC at 760 mmHg | |
| Melting Point | 148-151° | |
| LogP | 1.641 | |
| Hydrogen Bond Donor Count | 4 | |
| Hydrogen Bond Acceptor Count | 12 | |
| Rotatable Bond Count | 9 | |
| Heavy Atom Count | 38 | |
| Complexity | 460 | |
| Defined Atom Stereocenter Count | 0 | |
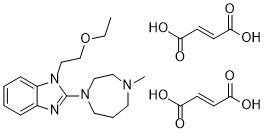
| SMILES | C(/C(=O)O)=C\C(=O)O.C(N1C2C=CC=CC=2N=C1N1CCN(C)CCC1)COCC |
|
| InChi Key | FWLKKPKZQYVAFR-LVEZLNDCSA-N | |
| InChi Code | InChI=1S/C17H26N4O.2C4H4O4/c1-3-22-14-13-21-16-8-5-4-7-15(16)18-17(21)20-10-6-9-19(2)11-12-20;2*5-3(6)1-2-4(7)8/h4-5,7-8H,3,6,9-14H2,1-2H3;2*1-2H,(H,5,6)(H,7,8)/b;2*2-1+ | |
| Chemical Name |
|
|
| Synonyms |
|
|
| HS Tariff Code | 2934.99.9001 | |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | H1 Receptor ( IC50 = 1.3 nM ); H2 Receptor ( IC50 = 49067 nM ); H3 Receptor ( IC50 = 12430 nM ) | |
| ln Vitro |
|
|
| ln Vivo |
|
|
| Enzyme Assay | Emedastine was significantly weaker at H2- (K1 = 49,067 +/- 11,113 nM) and H3- (Ki = 12,430 +/- 1,282 nM) receptors, but showed the highest affinity for H1-receptors (dissociation constant, Ki = 1.3 +/- 0.1 nM). The results showed that emedastine is a highly selective H1-receptor antagonist, with ratios of 37744, 9562, and 4 for H2:H1, H3:H1, and H2:H3 receptor affinities, respectively. Emedastine's H1-selectivity was significantly higher than pyrilamine's (H2:H1, H3:H1, and H2:H3 ratios of 11887, 12709, and 1, respectively). The antihistamines ketotifen (858, 1752, 0.5), levocabastine (420, 82, 5), pheniramine (430, 312, 1), chlorpheniramine (5700, 2216, 3), and antazoline (1163, 1110, 1) also demonstrated a notable lack of H1 selectivity in comparison to emedastine. Mededastine's ability to counteract histamine-induced phosphoinositide turnover in human trabecular meshwork cells was found to be potent (IC50 = 1.44 +/- 0.3 nM), which was in good agreement with its affinity for binding the H1 receptor site. These findings show that the most selective histamine antagonist for the H1-histamine receptor is emedastine, a histamine antagonist with high affinity and potency. | |
| Animal Protocol |
Male ICR mice 5-6 weeks of age 0.03, 0.1, 0.3 mg/kg Orally; 30 min before pruritogen injection |
|
| Toxicity/Toxicokinetics |
Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation Emedastine is an antihistamine that is not approved for marketing in the United States by the U.S. Food and Drug Administration, but is available in other countries. Preliminary data indicate that oral administration of 2 mg daily produces low levels in milk and does not affect the breastfed infant. When used as an eye drop, emedastine would not be expected to cause any adverse effects in breastfed infants. To substantially diminish the amount of drug that reaches the breastmilk after using eye drops, place pressure over the tear duct by the corner of the eye for 1 minute or more, then remove the excess solution with an absorbent tissue. ◉ Effects in Breastfed Infants A woman was prescribed emedastine difumarate 2 mg once daily and pranlukast hydrate 112.5 mg twice daily during pregnancy and postpartum. Her infant was breastfed and no adverse effects were noted. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. |
|
| References |
[1]. Emedastine: a potent, high affinity histamine H1-receptor-selective antagonist for ocular use: receptor binding and second messenger studies. J Ocul Pharmacol. 1994 Winter;10(4):653-64. [2]. Emedastine difumarate: a review of its potential ameliorating effect for tissue remodeling in allergic diseases. Expert Opin Pharmacother. 2009 Aug;10(11):1859-67. [3]. Involvement of blockade of leukotriene B(4) action in anti-pruritic effects of emedastine in mice. Eur J Pharmacol. 2000 Oct 6;406(1):149-52. |
|
| Additional Infomation |
Emedastine Difumarate is the difumarate salt form of emedastine, a second generation, selective histamine H1 receptor antagonist with anti-allergic activity. Emedastine reversibly and competitively blocks histamine by binding to H1 receptors, thus blocking its downstream activity. As a result, this agent interferes with mediator release from mast cells either by inhibiting calcium ion influx across mast cell/basophil plasma membrane or by inhibiting intracellular calcium ion release within the cells. In addition, emedastine may also inhibit the late-phase allergic reaction mediated through leukotrienes or prostaglandins, or by producing an anti-platelet activating factor effect. Upon ocular administration, emedastine causes a dose-dependent inhibition of histamine-stimulated vascular permeability in the conjunctiva. Emedastine does not affect adrenergic, dopamine, or serotonin receptors. Drug Indication Symptomatic treatment of seasonal allergic conjunctivitis. |
Solubility Data
| Solubility (In Vitro) |
|
|||
| Solubility (In Vivo) |
Note: Listed below are some common formulations that may be used to formulate products with low water solubility (e.g. < 1 mg/mL), you may test these formulations using a minute amount of products to avoid loss of samples. Injection Formulations (e.g. IP/IV/IM/SC) Injection Formulation 1: DMSO : Tween 80: Saline = 10 : 5 : 85 (i.e. 100 μL DMSO stock solution → 50 μL Tween 80 → 850 μL Saline) *Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH ₂ O to obtain a clear solution. Injection Formulation 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL DMSO → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Injection Formulation 3: DMSO : Corn oil = 10 : 90 (i.e. 100 μL DMSO → 900 μL Corn oil) Example: Take the Injection Formulation 3 (DMSO : Corn oil = 10 : 90) as an example, if 1 mL of 2.5 mg/mL working solution is to be prepared, you can take 100 μL 25 mg/mL DMSO stock solution and add to 900 μL corn oil, mix well to obtain a clear or suspension solution (2.5 mg/mL, ready for use in animals). Injection Formulation 4: DMSO : 20% SBE-β-CD in saline = 10 : 90 [i.e. 100 μL DMSO → 900 μL (20% SBE-β-CD in saline)] *Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Injection Formulation 5: 2-Hydroxypropyl-β-cyclodextrin : Saline = 50 : 50 (i.e. 500 μL 2-Hydroxypropyl-β-cyclodextrin → 500 μL Saline) Injection Formulation 6: DMSO : PEG300 : castor oil : Saline = 5 : 10 : 20 : 65 (i.e. 50 μL DMSO → 100 μLPEG300 → 200 μL castor oil → 650 μL Saline) Injection Formulation 7: Ethanol : Cremophor : Saline = 10: 10 : 80 (i.e. 100 μL Ethanol → 100 μL Cremophor → 800 μL Saline) Injection Formulation 8: Dissolve in Cremophor/Ethanol (50 : 50), then diluted by Saline Injection Formulation 9: EtOH : Corn oil = 10 : 90 (i.e. 100 μL EtOH → 900 μL Corn oil) Injection Formulation 10: EtOH : PEG300:Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL EtOH → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Oral Formulations Oral Formulation 1: Suspend in 0.5% CMC Na (carboxymethylcellulose sodium) Oral Formulation 2: Suspend in 0.5% Carboxymethyl cellulose Example: Take the Oral Formulation 1 (Suspend in 0.5% CMC Na) as an example, if 100 mL of 2.5 mg/mL working solution is to be prepared, you can first prepare 0.5% CMC Na solution by measuring 0.5 g CMC Na and dissolve it in 100 mL ddH2O to obtain a clear solution; then add 250 mg of the product to 100 mL 0.5% CMC Na solution, to make the suspension solution (2.5 mg/mL, ready for use in animals). Oral Formulation 3: Dissolved in PEG400 Oral Formulation 4: Suspend in 0.2% Carboxymethyl cellulose Oral Formulation 5: Dissolve in 0.25% Tween 80 and 0.5% Carboxymethyl cellulose Oral Formulation 6: Mixing with food powders Note: Please be aware that the above formulations are for reference only. InvivoChem strongly recommends customers to read literature methods/protocols carefully before determining which formulation you should use for in vivo studies, as different compounds have different solubility properties and have to be formulated differently. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.8707 mL | 9.3535 mL | 18.7070 mL | |
| 5 mM | 0.3741 mL | 1.8707 mL | 3.7414 mL | |
| 10 mM | 0.1871 mL | 0.9353 mL | 1.8707 mL |