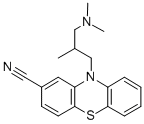
Cyamemazine (Tercian; Cianatil; cyamepromazine) is a typical antipsychotic drug of the phenothiazine class. It has been utilized to lessen the symptoms of ethanol withdrawal and as an anxiolytic antipsychotic.
Physicochemical Properties
| Molecular Formula | C19H21N3S |
| Molecular Weight | 323.45 |
| Exact Mass | 323.145 |
| Elemental Analysis | C, 70.55; H, 6.54; N, 12.99; S, 9.91 |
| CAS # | 3546-03-0 |
| Related CAS # | Cyamemazine-d6; 1216608-24-0 |
| PubChem CID | 62865 |
| Appearance | Solid powder |
| Density | 1.2±0.1 g/cm3 |
| Boiling Point | 479.0±45.0 °C at 760 mmHg |
| Melting Point | 204-205 |
| Flash Point | 243.5±28.7 °C |
| Vapour Pressure | 0.0±1.2 mmHg at 25°C |
| Index of Refraction | 1.655 |
| LogP | 4.62 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 4 |
| Heavy Atom Count | 23 |
| Complexity | 443 |
| Defined Atom Stereocenter Count | 0 |
| SMILES | CC(CN(C)C)CN1C2=CC=CC=C2SC3=C1C=C(C=C3)C#N |
| InChi Key | SLFGIOIONGJGRT-UHFFFAOYSA-N |
| InChi Code | InChI=1S/C19H21N3S/c1-14(12-21(2)3)13-22-16-6-4-5-7-18(16)23-19-9-8-15(11-20)10-17(19)22/h4-10,14H,12-13H2,1-3H3 |
| Chemical Name | 10-[3-(dimethylamino)-2-methylpropyl]phenothiazine-2-carbonitrile |
| Synonyms | Cyamepromazine; TercianCianatil; Cyamemazine |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | 5-HT2A Receptor ( Ki = 1.5 nM ); 5-HT2C Receptor ( Ki = 12 nM ); 5-HT3 Receptor ( Ki = 75 nM ) |
| ln Vitro | Cyamemazine's antipsychotic action is consistent with its high affinity for dopamine receptors. Cyamemazine's affinity for M1 (Ki = 13 nM), M2 (Ki = 42 nM), M3 (Ki = 321 nM), M4 (Ki = 12 nM), and M5 (Ki = 35 nM) receptors is consistent with its antagonist activity at muscarinic receptors[1]. |
| ln Vivo | Cyamemazine functions as an antagonist at the 5-HT3, 5-HT2C, and 5-HT2A receptors in the rat brain membrane in the 5-HT2C-dependent phospholipase C stimulation, in the 5-HT3-dependent contraction of the isolated guinea pig ileum and bradycardic responses, and in the 5-HT2A-dependent contraction of the isolated rat aorta rings and the isolated guinea pig trachea. The antagonism of 5-HT3 and 5-HT2C receptors by cyamemazine is partly responsible for its therapeutic action against anxiety disorders. Low doses of cyclomazine administered acutely can lower metabolite and extracellular dopamine concentrations in the rat striatum[1]. |
| References |
[1]. Preclinical and clinical pharmacology of cyamemazine: anxiolytic effects and prevention of alcohol and benzodiazepine withdrawal syndrome. CNS Drug Rev. 2004 Fall;10(3):219-29. [2]. Photobehavior of the antipsychotic drug cyamemazine in a supramolecular gel protective environment. J Photochem Photobiol B. 2020 Jan;202:111686. |
| Additional Infomation |
Cyamemazine is a member of phenothiazines. Cyamemazine (Tercian), also known as cyamepromazine, is a typical antipsychotic drug of the phenothiazine class used primarily in the treatment of schizophrenia and psychosis-associated anxiety. Cyamemazine actually behaves like an atypical antipsychotic, due to its potent anxiolytic effects (5-HT2C) and lack of extrapyramidal side effects (5-HT2A). |
Solubility Data
| Solubility (In Vitro) | DMSO: ~100 mg/mL (~309.2 mM) |
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.5 mg/mL (7.73 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: 2.5 mg/mL (7.73 mM) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), suspension solution; with ultrasonication. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 3: ≥ 2.5 mg/mL (7.73 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.0917 mL | 15.4583 mL | 30.9167 mL | |
| 5 mM | 0.6183 mL | 3.0917 mL | 6.1833 mL | |
| 10 mM | 0.3092 mL | 1.5458 mL | 3.0917 mL |