Physicochemical Properties
| Molecular Formula | C26H28O13 |
| Exact Mass | 548.152 |
| CAS # | 185145-33-9 |
| PubChem CID | 21722008 |
| Appearance | Typically exists as solid at room temperature |
| LogP | -1.8 |
| Hydrogen Bond Donor Count | 9 |
| Hydrogen Bond Acceptor Count | 13 |
| Rotatable Bond Count | 4 |
| Heavy Atom Count | 39 |
| Complexity | 908 |
| Defined Atom Stereocenter Count | 9 |
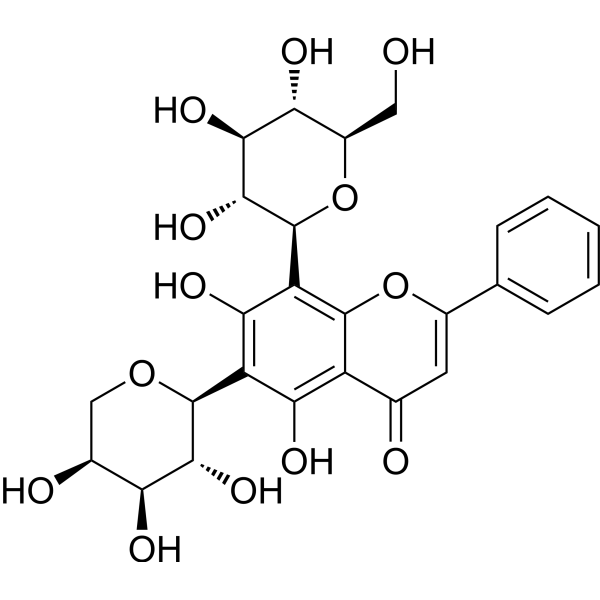
| SMILES | C1[C@@H]([C@@H]([C@H]([C@@H](O1)C2=C(C(=C3C(=C2O)C(=O)C=C(O3)C4=CC=CC=C4)[C@H]5[C@@H]([C@H]([C@@H]([C@H](O5)CO)O)O)O)O)O)O)O |
| InChi Key | NZZNHGSHLAHPCG-VYUBKLCTSA-N |
| InChi Code | InChI=1S/C26H28O13/c27-7-13-18(31)21(34)23(36)26(39-13)16-20(33)15(25-22(35)17(30)11(29)8-37-25)19(32)14-10(28)6-12(38-24(14)16)9-4-2-1-3-5-9/h1-6,11,13,17-18,21-23,25-27,29-36H,7-8H2/t11-,13+,17-,18+,21-,22+,23+,25-,26-/m0/s1 |
| Chemical Name | 5,7-dihydroxy-2-phenyl-8-[(2S,3R,4R,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]-6-[(2S,3R,4S,5S)-3,4,5-trihydroxyoxan-2-yl]chromen-4-one |
| Synonyms | Chrysin 6-C-arabinoside 8-C-glucoside; 185145-33-9; 5,7-dihydroxy-2-phenyl-8-[(2S,3R,4R,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]-6-[(2S,3R,4S,5S)-3,4,5-trihydroxyoxan-2-yl]chromen-4-one; AKOS040760325; Chrysina6-C-arabinosidea8-C-glucoside; FS-7639; E87098; 5,7-Dihydroxy-2-phenyl-8-((2S,3R,4R,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)tetrahydro-2H-pyran-2-yl)-6-((2S,3R,4S,5S)-3,4,5-trihydroxytetrahydro-2H-pyran-2-yl)-4H-chromen-4-one |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | α±-glucosidase |
| ln Vitro | Twenty-six common peaks were assigned and identified from the fingerprints of different proportions DJS extracts. In vitro experimental results showed that DJS extracts inhibited inflammation and release of CGRP from trigeminal nerve cells. Five predicted active compounds, Chrysin 6-C-arabinoside 8-C-glucoside, Chrysin 6-C-glucoside 8-C-arabinoside, baicalin, Chrysin-7-O-Beta-D-glucoronide and Oroxylin A 7-O-glucuronide were sorted out according to spectrum-effect relationship analysis and molecular docking comprehensively. In vitro validation experiments showed that all the predicted compounds inhibited the CGRP releasing and the activation of TRPV1 channel. Baicalin, chrysin-7-O-β-D-glucuronide and Oroxylin A-7-glucoronide significantly inhibited the activation of TRPV1 channel. Conclusion: Chrysin 6-C-arabinoside 8-C-glucoside, Chrysin 6-C-glucoside 8-C-arabinoside, baicalin, Chrysin-7-O-Beta-D-glucoronide and Oroxylin A 7-O-glucuronide which can inhibit the CGRP releasing and the activation of TRPV1 channel were screened as the anti-migraine active compounds by spectrum-effect relationship analysis and molecular docking. https://pubmed.ncbi.nlm.nih.gov/34161797/ |
| Enzyme Assay | Aim of the study: This study aimed to uncover the anti-migraine active compounds from DJS and preliminary predicted the pharmacological mechanism by evaluating the spectrum-effect relationship between high-performance liquid chromatography (HPLC) fingerprints and anti-migraine effects of Duijinsan (DJS) extract combined with molecular docking. Materials and methods: HPLC and LC-MS were applied for chemical analyses of DJS extracts in different proportions. Inhibition of DJS extracts on trigeminal nerve cell releasing calcitonin gene related peptide (CGRP) experiment was performed. The active compounds were screened by spectrum-effect relationship analysis and confirmed by molecular docking and the activities of major predicted compounds were validated in vitro. https://pubmed.ncbi.nlm.nih.gov/34161797/ |
| References |
[1]. Efficient purification of flavonoids from bamboo shoot residues of Phyllostachys edulis by macroporous resin and their hypoglycemic activity. Food Chemistry: X. |
| Additional Infomation | Chrysin 6-C-arabinoside 8-C-glucoside has been reported in Scutellaria baicalensis with data available. |
Solubility Data
| Solubility (In Vitro) | May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples |
| Solubility (In Vivo) |
Note: Listed below are some common formulations that may be used to formulate products with low water solubility (e.g. < 1 mg/mL), you may test these formulations using a minute amount of products to avoid loss of samples. Injection Formulations (e.g. IP/IV/IM/SC) Injection Formulation 1: DMSO : Tween 80: Saline = 10 : 5 : 85 (i.e. 100 μL DMSO stock solution → 50 μL Tween 80 → 850 μL Saline) *Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH ₂ O to obtain a clear solution. Injection Formulation 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL DMSO → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Injection Formulation 3: DMSO : Corn oil = 10 : 90 (i.e. 100 μL DMSO → 900 μL Corn oil) Example: Take the Injection Formulation 3 (DMSO : Corn oil = 10 : 90) as an example, if 1 mL of 2.5 mg/mL working solution is to be prepared, you can take 100 μL 25 mg/mL DMSO stock solution and add to 900 μL corn oil, mix well to obtain a clear or suspension solution (2.5 mg/mL, ready for use in animals). Injection Formulation 4: DMSO : 20% SBE-β-CD in saline = 10 : 90 [i.e. 100 μL DMSO → 900 μL (20% SBE-β-CD in saline)] *Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Injection Formulation 5: 2-Hydroxypropyl-β-cyclodextrin : Saline = 50 : 50 (i.e. 500 μL 2-Hydroxypropyl-β-cyclodextrin → 500 μL Saline) Injection Formulation 6: DMSO : PEG300 : castor oil : Saline = 5 : 10 : 20 : 65 (i.e. 50 μL DMSO → 100 μLPEG300 → 200 μL castor oil → 650 μL Saline) Injection Formulation 7: Ethanol : Cremophor : Saline = 10: 10 : 80 (i.e. 100 μL Ethanol → 100 μL Cremophor → 800 μL Saline) Injection Formulation 8: Dissolve in Cremophor/Ethanol (50 : 50), then diluted by Saline Injection Formulation 9: EtOH : Corn oil = 10 : 90 (i.e. 100 μL EtOH → 900 μL Corn oil) Injection Formulation 10: EtOH : PEG300:Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL EtOH → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Oral Formulations Oral Formulation 1: Suspend in 0.5% CMC Na (carboxymethylcellulose sodium) Oral Formulation 2: Suspend in 0.5% Carboxymethyl cellulose Example: Take the Oral Formulation 1 (Suspend in 0.5% CMC Na) as an example, if 100 mL of 2.5 mg/mL working solution is to be prepared, you can first prepare 0.5% CMC Na solution by measuring 0.5 g CMC Na and dissolve it in 100 mL ddH2O to obtain a clear solution; then add 250 mg of the product to 100 mL 0.5% CMC Na solution, to make the suspension solution (2.5 mg/mL, ready for use in animals). Oral Formulation 3: Dissolved in PEG400 Oral Formulation 4: Suspend in 0.2% Carboxymethyl cellulose Oral Formulation 5: Dissolve in 0.25% Tween 80 and 0.5% Carboxymethyl cellulose Oral Formulation 6: Mixing with food powders Note: Please be aware that the above formulations are for reference only. InvivoChem strongly recommends customers to read literature methods/protocols carefully before determining which formulation you should use for in vivo studies, as different compounds have different solubility properties and have to be formulated differently. (Please use freshly prepared in vivo formulations for optimal results.) |