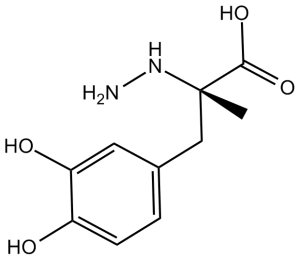
Carbidopa (also known as MK485, MK-485; trade name: Lodosyn; abbreviated as CD), a potent and competitive inhibitor of aromatic l-amino acid decarboxylase that does not cross the blood-brain barrier, is routinely administered with levodopa (LD) to patients with Parkinson disease (PD) to reduce the peripheral decarboxylation of LD to dopamine. CD premedication improves 11C-5-HTP PET image quality and facilitates detection of NET lesions. Because of the similarity of metabolic pathways, this method could probably be applied to improve PET imaging using other tracers like 18F-DOPA and 11C-DOPA. Carbidopa (100 microM) decreased growth of (but did not kill) SK-N-SH neuroblastoma and A204 rhabdomyosarcoma cells and did not affect proliferation of DU 145 prostate, MCF7 breast, or NCI-H460 large cell lung carcinoma lines. sublethal doses of carbidopa produced additive cytotoxic effects in carcinoid cells in combination with etoposide and cytotoxic synergy in SCLC cells when coincubated with topotecan.
Physicochemical Properties
| Molecular Formula | C10H14N2O4 | |
| Molecular Weight | 226.23 | |
| Exact Mass | 226.095 | |
| CAS # | 28860-95-9 | |
| Related CAS # | Carbidopa monohydrate;38821-49-7 | |
| PubChem CID | 34359 | |
| Appearance | White to off-white solid powder | |
| Density | 1.4±0.1 g/cm3 | |
| Boiling Point | 528.7±50.0 °C at 760 mmHg | |
| Melting Point | 206 - 208ºC | |
| Flash Point | 273.5±30.1 °C | |
| Vapour Pressure | 0.0±1.5 mmHg at 25°C | |
| Index of Refraction | 1.641 | |
| LogP | -0.19 | |
| Hydrogen Bond Donor Count | 5 | |
| Hydrogen Bond Acceptor Count | 6 | |
| Rotatable Bond Count | 4 | |
| Heavy Atom Count | 16 | |
| Complexity | 261 | |
| Defined Atom Stereocenter Count | 1 | |
| SMILES | C[C@](CC1=CC(=C(C=C1)O)O)(C(=O)O)NN |
|
| InChi Key | TZFNLOMSOLWIDK-JTQLQIEISA-N | |
| InChi Code | InChI=1S/C10H14N2O4/c1-10(12-11,9(15)16)5-6-2-3-7(13)8(14)4-6/h2-4,12-14H,5,11H2,1H3,(H,15,16)/t10-/m0/s1 | |
| Chemical Name | (2S)-3-(3,4-dihydroxyphenyl)-2-hydrazinyl-2-methylpropanoic acid | |
| Synonyms |
|
|
| HS Tariff Code | 2934.99.9001 | |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| ln Vitro | In B\PC3 and Capan-2 cells, carbidetopa ((S)-(-)-Carbidopa) demonstrates actions akin to those reported for other AhR ligands, including the activation of CYP1A1 and CYP1A2, which are inhibited by AhR single antioxidants like CH223191 [1]. |
| ln Vivo | In vivo investigations employing Bχ PC3 cells as xenografts have demonstrated that carbidopa at a dose of 1 mg/ml greatly reduces tumor growth. Carbidopa also promotes the nuclear envelope of AhR [1]. |
| ADME/Pharmacokinetics |
Absorption, Distribution and Excretion When [levodopa]/carbidopa is administered orally, 40-70% of the administered dose is absorbed. Once absorbed, carbidopa shows bioavailability of 58%. A maximum concentration of 0.085 mcg/ml was achieved after 143 min with an AUC of 19.28 mcg.min/ml. In animal studies, 66% of the administered dose of carbidopa was eliminated via the urine while 11% was found in feces. These studies were performed in humans and it was observed a urine excretion covering 50% of the administered dose. The volume of distribution reported for the combination therapy of carbidopa/[levodopa] is of 3.6 L/kg. However, carbidopa is widely distributed in the tissues, except in the brain. After one hour, carbidopa is found mainly in the kidney, lungs, small intestine and liver. The reported clearance rate for the combination therapy of [levodopa]/carbidopa is 51.7 L/h. Metabolism / Metabolites The loss of the hydrazine functional group (probably as molecular nitrogen) represents the major metabolic pathway for carbidopa. There are several metabolites of carbidopa metabolism including 3-(3,4-dihydroxyphenyl)-2-methylpropionic acid, 3-(4-hydroxy-3-methoxyphenyl)-2-methylpropionic acid, 3-(3-hydroxyphenyl)-2-methylpropionic acid, 3-(4-hydroxy-3-methoxyphenyl)-2-methyllactic acid, 3-(3-hydroxyphenyl)-2-methyllactic acid, and 3,4-dihydroxyphenylacetone (1,2). Biological Half-Life The reported half-life of carbidopa is of approximately 107 minutes. |
| Toxicity/Toxicokinetics |
Protein Binding It is widely accepted that the protein binding of carbidopa is 76%. However, more studies are required or the presentation of the source of this information. |
| References |
[1]. Safe S. Carbidopa: a selective Ah receptor modulator (SAhRM). Biochem J. 2017;474(22):3763-3765. Published 2017 Nov 6. [2]. Fermaglich J. Treatment of Parkinson's disease with carbidopa, a peripheral decarboxylase inhibitor, and levodopa. Med Ann Dist Columbia. 1974;43(12):587-591. |
| Additional Infomation |
Pharmacodynamics When mixed with [levodopa], carbidopa inhibits the peripheral conversion of [levodopa] to dopamine and the decarboxylation of [oxitriptan] to serotonin by aromatic L-amino acid decarboxylase. This results in an increased amount of [levodopa] and [oxitriptan] available for transport to the central nervous system. Carbidopa also inhibits the metabolism of [levodopa] in the GI tract, thus, increasing the bioavailability of [levodopa]. The presence of additional units of circulating [levodopa] can increase the effectiveness of the still functional dopaminergic neurons and it has been shown to alleviate symptoms for a time. The action of carbidopa is very important as [levodopa] is able to cross the blood-brain barrier while dopamine cannot. Hence the administration of carbidopa is essential to prevent the transformation of external [levodopa] to dopamine before reaching the main action site in the brain. The coadministration of carbidopa with [levodopa] has been shown to increase the half-life of [levodopa] more than 1.5 times while increasing the plasma level and decreasing clearance. The combination therapy has also shown an increase of the recovery of [levodopa] in urine instead of dopamine which proves a reduced metabolism. This effect has been highly observed by a significant reduction in [levodopa] requirements and a significant reduction in the presence of side effects such as nausea. It has been observed that the effect of carbidopa is not dose-dependent. |
Solubility Data
| Solubility (In Vitro) |
|
|||
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 1 mg/mL (4.42 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 10.0 mg/mL clear DMSO stock solution to 400 μL of PEG300 and mix evenly; then add 50 μL of Tween-80 to the above solution and mix evenly; then add 450 μL of normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 1 mg/mL (4.42 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 10.0 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 3: ≥ 1 mg/mL (4.42 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 10.0 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. Solubility in Formulation 4: 10 mg/mL (44.20 mM) in 50% PEG300 50% Saline (add these co-solvents sequentially from left to right, and one by one), suspension solution; with ultrasonication. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 4.4203 mL | 22.1014 mL | 44.2028 mL | |
| 5 mM | 0.8841 mL | 4.4203 mL | 8.8406 mL | |
| 10 mM | 0.4420 mL | 2.2101 mL | 4.4203 mL |