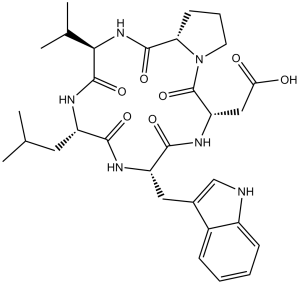
BQ-123 (Cyclo(D-trp-D-asp-pro-D-val-leu); BQ123; BQ 123) is a potent and selective antagonist of endothelin A receptor (ETAR) with an IC50 of 7.3 nM. It has been demonstrated that endothelin-1 increases the activity of neurons and glutaminergic synaptic transmission through endothelin-A receptors (ETAR) in the nucleus tractus solitarius neurons, which are crucial in epileptic seizures. Consequently, BQ-123, an ETAR antagonist, may reduce glutaminergic synaptic transmission and neuronal excitability. BQ123 decreases myocardial infarction after focal cerebral ischemia in rats and amplifies hypothermia induced by acetaminophen. In LPS-treated rats, BQ123 Activates Nrf2 to Stimulate Skeletal Muscle Antioxidant Defense. Following surgery for congenital heart disease, BQ123 lowers pulmonary vascular resistance. BQ123 shields against endothelial and myocardial reperfusion damage.
Physicochemical Properties
| Molecular Formula | C31H42N6O7 | |
| Molecular Weight | 610.7 | |
| Exact Mass | 610.311 | |
| Elemental Analysis | C, 60.97; H, 6.93; N, 13.76; O, 18.34 | |
| CAS # | 136553-81-6 | |
| Related CAS # | BQ-123 TFA | |
| PubChem CID | 443289 | |
| Appearance | White to off-white solid powder | |
| Density | 1.3±0.1 g/cm3 | |
| Boiling Point | 1053.6±65.0 °C at 760 mmHg | |
| Flash Point | 591.0±34.3 °C | |
| Vapour Pressure | 0.0±0.3 mmHg at 25°C | |
| Index of Refraction | 1.621 | |
| LogP | -0.1 | |
| Hydrogen Bond Donor Count | 6 | |
| Hydrogen Bond Acceptor Count | 7 | |
| Rotatable Bond Count | 7 | |
| Heavy Atom Count | 44 | |
| Complexity | 1110 | |
| Defined Atom Stereocenter Count | 5 | |
| SMILES | O=C(N[C@](C(N(CCC1)[C@]1([H])C2=O)=O)([H])CC(O)=O)[C@H](NC([C@@H](NC([C@H](N2)C(C)C)=O)CC(C)C)=O)CC3=CNC4=CC=CC=C34 |
|
| InChi Key | VYCMAAOURFJIHD-PJNXIOHISA-N | |
| InChi Code | InChI=1S/C31H42N6O7/c1-16(2)12-21-27(40)33-22(13-18-15-32-20-9-6-5-8-19(18)20)28(41)35-23(14-25(38)39)31(44)37-11-7-10-24(37)29(42)36-26(17(3)4)30(43)34-21/h5-6,8-9,15-17,21-24,26,32H,7,10-14H2,1-4H3,(H,33,40)(H,34,43)(H,35,41)(H,36,42)(H,38,39)/t21-,22+,23+,24-,26+/m0/s1 | |
| Chemical Name | 2-[(3R,6R,9S,12R,15S)-6-(1H-indol-3-ylmethyl)-9-(2-methylpropyl)-2,5,8,11,14-pentaoxo-12-propan-2-yl-1,4,7,10,13-pentazabicyclo[13.3.0]octadecan-3-yl]acetic acid | |
| Synonyms |
|
|
| HS Tariff Code | 2934.99.9001 | |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month Note: Please store this product in a sealed and protected environment (e.g. under nitrogen), avoid exposure to moisture and light. |
|
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | Endothelin A receptor ( IC50 = 7.3 nM ) | ||
| ln Vitro |
|
||
| ln Vivo |
|
||
| Enzyme Assay | BQ-123 is a potent and selective endothelin A receptor (ETAR) antagonist with IC50 of 7.3 nM. | ||
| Cell Assay | BQ123 (10-6 M) blocked endothelin-1 (ET-1) (10-6 M)-induced [Ca2+]i increase in ETA-expressing cells by 95%. BQ-123 prevented ET-1-induced cellular contraction, [Ca2+ ]i mobilization, [3H]thymidine incorporation, MAP kinase activation, and MTT reduction in rat vascular smooth muscle cells (VSMC). However, MAP kinase activity and [Ca2+]i mobilization were not inhibited by BQ-123 in response to angiotensin II (Ang II) or arginine vasopressin (AVP). | ||
| Animal Protocol |
|
||
| References |
[1]. J Cardiovasc Pharmacol . 1992:20 Suppl 12:S11-4. [2]. FEBS Lett . 1992 May 18;302(3):243-6. [3]. Biomed Pharmacother . 2010 Nov;64(9):639-46. [4]. Hum Exp Toxicol . 2014 Oct;33(10):1008-16. [5]. Toxicol Appl Pharmacol . 2015 Feb 1;282(3):275-84. |
||
| Additional Infomation |
BQ 123 is a cyclic peptide. BQ-123 has been investigated for the basic science and treatment of Coronary Artery Disease, Aorto-coronary Bypass Grafting, and ST-Elevation Myocardial Infarction. |
Solubility Data
| Solubility (In Vitro) |
|
|||
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 5 mg/mL (8.19 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 50.0 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 5 mg/mL (8.19 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 50.0 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 3: ≥ 5 mg/mL (8.19 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 50.0 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.6375 mL | 8.1873 mL | 16.3747 mL | |
| 5 mM | 0.3275 mL | 1.6375 mL | 3.2749 mL | |
| 10 mM | 0.1637 mL | 0.8187 mL | 1.6375 mL |