Avatrombopag (formerly AKR-501, YM-477, AS-1670542; E-5501; trade name: Doptelet) is an orally-active small molecule thrombopoietin (TPO) receptor agonist that was used for the first time in 2008 to treat patients with chronic idiopathic thrombocytopenic purpura. Thrombopoietin (TPO) is the principal physiologic regulator of platelet production. As of May 2018, Eltrombopag was approved by US FDA to treat low blood platelet count (thrombocytopenia) in adults with chronic liver disease who are scheduled to undergo a medical or dental procedure. AKR-501 specifically targeted the TPO receptor and stimulated megakaryocytopoiesis throughout the development and maturation of megakaryocytes just as rhTPO did. AKR-501, however, was shown to be effective only in humans and chimpanzees with high species specificity.
Physicochemical Properties
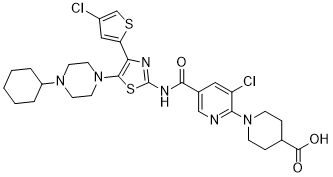
| Molecular Formula | C29H34CL2N6O3S2 | |
| Molecular Weight | 649.65 | |
| Exact Mass | 648.151 | |
| CAS # | 570406-98-3 | |
| Related CAS # | Avatrombopag hydrochloride;570403-17-7;Avatrombopag maleate;677007-74-8 | |
| PubChem CID | 9852519 | |
| Appearance | White to off-white solid powder | |
| Density | 1.4±0.1 g/cm3 | |
| Index of Refraction | 1.671 | |
| LogP | 6.82 | |
| Hydrogen Bond Donor Count | 2 | |
| Hydrogen Bond Acceptor Count | 10 | |
| Rotatable Bond Count | 7 | |
| Heavy Atom Count | 42 | |
| Complexity | 935 | |
| Defined Atom Stereocenter Count | 0 | |
| InChi Key | OFZJKCQENFPZBH-UHFFFAOYSA-N | |
| InChi Code | InChI=1S/C29H34Cl2N6O3S2/c30-20-15-23(41-17-20)24-27(37-12-10-35(11-13-37)21-4-2-1-3-5-21)42-29(33-24)34-26(38)19-14-22(31)25(32-16-19)36-8-6-18(7-9-36)28(39)40/h14-18,21H,1-13H2,(H,39,40)(H,33,34,38) | |
| Chemical Name |
|
|
| Synonyms |
|
|
| HS Tariff Code | 2934.99.9001 | |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| ln Vitro | As with recombinant human TPO (rhTPO), avatrombopag (E5501; AKR-501) specifically binds the TPO receptor and induces megakaryopoiesis throughout megakaryocyte development and maturation [1]. In a concentration-dependent way, avatrombopag (0-100 nM) promotes the growth of Ba/F3 cells that express TPO receptors. Similar to rhTPO, avatrombopag (0-3 μM) causes tyrosine phosphorylation of STAT3 and STAT5, as well as threonine phosphorylation of ERK, in cells [1]. In human CD34+ cells, avatrombopag stimulates the development of megakaryocyte colonies in a concentration-dependent way. Avatrombopag has a 25 nM EC50 and a maximal activity that is comparable to rhTPO [1]. |
| ln Vivo | NOD/SCID mice transplanted with human FL CD34+ cells have higher human platelet counts when administered with avatrombopag (0.3-3 mg/kg; oral; daily for 14 days) [1]. |
| Cell Assay |
Cell proliferation assay [1] Cell Types: Ba/F3 cells Tested Concentrations: 0.003 μM, 0.03 μM, 0.3 μM, 3 μM Incubation Duration: Experimental Results: Increased proliferation of Ba/F3 cells expressing TPO receptor in a concentration-dependent manner. Western Blot Analysis [1] Cell Types: Ba/F3 cells Tested Concentrations: 0.003 μM, 0.03 μM, 0.3 μM, 3 μM Incubation Duration: 15 minutes Experimental Results: Induction of intracellular tyrosine phosphorylation of STAT3 and STAT5 and threonine of ERK Acid phosphorylation. |
| Animal Protocol |
Animal/Disease Models: NOD/SCID (severe combined immunodeficient) mouse (transplanted with human FL CD34+ cells) [1] Doses: 0.3, 1, and 3 mg/kg Route of Administration: Po; one time/day for 14 days Experimental Results: Human platelet counts were dose-dependent The increase was approximately 2.7-fold at 1 mg/kg/d and approximately 3.0-fold at 3 mg/kg/d on day 14 after initiation of administration. |
| ADME/Pharmacokinetics |
Absorption, Distribution and Excretion Following single dosing under fasted and fed conditions, mean peak concentrations occurred at 5-8 hours and declined with a half-life of 16-18 hours in Japanese and white subjects. Administration with food did not have an effect on the rate or extent of avatrombopag absorption, however, significantly reduced pharmacokinetic variability relative to the fasting state. Avatrombopag showed dose-proportional pharmacokinetics after single doses from 10 mg (0.25-times the lowest approved dosage) to 80 mg (1.3-times the highest recommended dosage). Healthy subjects administered 40 mg of avatrombopag showed a geometric mean (%CV) maximal concentration (Cmax) of 166 (84%) ng/mL and area under the time-concentration curve, extrapolated to infinity (AUC0-inf) of 4198 (83%) ng.hr/mL. The pharmacokinetics of avatrombopag are similar in both healthy subjects and the chronic liver disease population. Fecal excretion accounted for 88% of the administered dose, with 34% of the dose excreted as unchanged avatrombopag. Only 6% of the administered dose was found in urine. Avatrombopag has an estimated mean volume of distribution (%CV) of 180 L (25%). The mean (%CV) of the clearance of avatrombopag is estimated to be 6.9 L/hr (29%). Metabolism / Metabolites Avatrombopag is primarily metabolized by CYP2C9 and CYP3A4. Biological Half-Life The mean plasma elimination half-life (%CV) of avatrombopag is approximately 19 hours (19%). |
| Toxicity/Toxicokinetics |
Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation No information is available on the use of avatrombopag during breastfeeding. The manufacturer recommends avoiding breastfeeding during the use of avatrombopag and for at least 2 weeks after the last dose. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Protein Binding Avatrombopag is greater than 96% bound to human plasma proteins. |
| References |
[1]. AKR-501 (YM477) a novel orally-active thrombopoietin receptor agonist. Eur J Haematol. 2009;82(4):247-254. [2]. Avatrombopag for the treatment of thrombocytopenia in patients with chronic liver disease. Expert Rev Clin Pharmacol. 2019 Sep;12(9):859-865. [3]. Pharmacokinetic/pharmacodynamic drug-drug interactions of avatrombopag when coadministered with dual or selective CYP2C9 and CYP3A interacting drugs. Br J Clin Pharmacol. 2018;84(5):952-960. |
| Additional Infomation |
Avatrombopag (Doptelet), is an orally administered, small molecule thrombopoietin receptor (c-Mpl) agonist that increases platelet number without increasing platelet activation, thereby decreasing the need for blood transfusions. Patients with thrombocytopenia and chronic liver disease often require platelet transfusions before surgical procedures to decrease the risk of bleeding. Thrombocytopenia is a common complication in patients suffering from chronic liver disease, occurring as a result of liver disease or a consequence of interferon-based antiviral therapy. Avatrombopag was first approved by the FDA in May 2018 for use in adults with chronic liver disease who are scheduled to undergo a procedure. It is administered orally as the salt form avatrombopag maleate. Doptelet (Avatrombopag) is the first orally administered treatment option for patients with chronic liver disease, allowing a large population of patients to avoid a platelet transfusion before a procedure by increasing platelet counts to the optimal level ≥50,000 per microliter. Avatrombopag is an orally available platelet thrombopoietin receptor (TPOR; MPL) agonist, with potential megakaryopoiesis stimulating activity. Upon administration, avatrombopag binds to and stimulates TPOR, which may lead to the proliferation and differentiation of megakaryocytes from bone marrow progenitor cells. This increases the production of platelets and may prevent chemotherapy-induced thrombocytopenia (CIT). TPOR is a cytokine receptor and member of the hematopoietin receptor superfamily. See also: Avatrombopag Maleate (active moiety of). Drug Indication Indicated for the treatment of thrombocytopenia in adult patients with chronic liver disease who are scheduled to undergo a procedure. It is also indicated in adult patients with chronic immune thrombocytopenia who have had an insufficient response to a previous treatment. FDA Label Doptelet is indicated for the treatment of severe thrombocytopenia in adult patients with chronic liver disease who are scheduled to undergo an invasive procedure. Doptelet is indicated for the treatment of primary chronic immune thrombocytopenia (ITP) in adult patients who are refractory to other treatments (e. g. corticosteroids, immunoglobulins). Treatment of chemotherapy-induced thrombocytopenia Mechanism of Action Avatrombopag is an orally bioavailable, small molecule thrombopoietin (TPO) receptor agonist that stimulates proliferation and differentiation of megakaryocytes from bone marrow progenitor cells resulting in an increased production of platelets. Avatrombopag is not competitive with thrombopoietin for binding to the TPO receptor and has an additive pharmacological effect with TPO on platelet production. Avatrombopag is a thrombopoietin receptor (TPOR; MPL) agonist, with possible megakaryopoiesis stimulating activity. After administration, avatrombopag binds to and stimulates the platelet thrombopoeitin receptor (TPOR), which can lead to the proliferation and differentiation of megakaryocytes from bone marrow progenitor cells. This process increases the production of platelets and may serve to prevent chemotherapy-induced thrombocytopenia (CIT). TPOR is classified as a cytokine receptor and as a member of the hematopoietin receptor superfamily. Pharmacodynamics In a study of efficacy, avatrombopag resulted in dose and exposure-dependent elevations in platelet counts in adults. The onset of the platelet count increase was noted within 3 to 5 days of the start of a 5-day treatment course, with the highest level of effect measured after 10 to 13 days. Following this, platelet counts decreased gradually, returning to near baseline values at the 35-day point. Increased platelet activation leads to increased blood clotting, which may lead to various complications. Avatrombopag does not lead to increased platelet activation. |
Solubility Data
| Solubility (In Vitro) |
|
|||
| Solubility (In Vivo) |
Note: Listed below are some common formulations that may be used to formulate products with low water solubility (e.g. < 1 mg/mL), you may test these formulations using a minute amount of products to avoid loss of samples. Injection Formulations (e.g. IP/IV/IM/SC) Injection Formulation 1: DMSO : Tween 80: Saline = 10 : 5 : 85 (i.e. 100 μL DMSO stock solution → 50 μL Tween 80 → 850 μL Saline) *Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH ₂ O to obtain a clear solution. Injection Formulation 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL DMSO → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Injection Formulation 3: DMSO : Corn oil = 10 : 90 (i.e. 100 μL DMSO → 900 μL Corn oil) Example: Take the Injection Formulation 3 (DMSO : Corn oil = 10 : 90) as an example, if 1 mL of 2.5 mg/mL working solution is to be prepared, you can take 100 μL 25 mg/mL DMSO stock solution and add to 900 μL corn oil, mix well to obtain a clear or suspension solution (2.5 mg/mL, ready for use in animals). Injection Formulation 4: DMSO : 20% SBE-β-CD in saline = 10 : 90 [i.e. 100 μL DMSO → 900 μL (20% SBE-β-CD in saline)] *Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Injection Formulation 5: 2-Hydroxypropyl-β-cyclodextrin : Saline = 50 : 50 (i.e. 500 μL 2-Hydroxypropyl-β-cyclodextrin → 500 μL Saline) Injection Formulation 6: DMSO : PEG300 : castor oil : Saline = 5 : 10 : 20 : 65 (i.e. 50 μL DMSO → 100 μLPEG300 → 200 μL castor oil → 650 μL Saline) Injection Formulation 7: Ethanol : Cremophor : Saline = 10: 10 : 80 (i.e. 100 μL Ethanol → 100 μL Cremophor → 800 μL Saline) Injection Formulation 8: Dissolve in Cremophor/Ethanol (50 : 50), then diluted by Saline Injection Formulation 9: EtOH : Corn oil = 10 : 90 (i.e. 100 μL EtOH → 900 μL Corn oil) Injection Formulation 10: EtOH : PEG300:Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL EtOH → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Oral Formulations Oral Formulation 1: Suspend in 0.5% CMC Na (carboxymethylcellulose sodium) Oral Formulation 2: Suspend in 0.5% Carboxymethyl cellulose Example: Take the Oral Formulation 1 (Suspend in 0.5% CMC Na) as an example, if 100 mL of 2.5 mg/mL working solution is to be prepared, you can first prepare 0.5% CMC Na solution by measuring 0.5 g CMC Na and dissolve it in 100 mL ddH2O to obtain a clear solution; then add 250 mg of the product to 100 mL 0.5% CMC Na solution, to make the suspension solution (2.5 mg/mL, ready for use in animals). Oral Formulation 3: Dissolved in PEG400 Oral Formulation 4: Suspend in 0.2% Carboxymethyl cellulose Oral Formulation 5: Dissolve in 0.25% Tween 80 and 0.5% Carboxymethyl cellulose Oral Formulation 6: Mixing with food powders Note: Please be aware that the above formulations are for reference only. InvivoChem strongly recommends customers to read literature methods/protocols carefully before determining which formulation you should use for in vivo studies, as different compounds have different solubility properties and have to be formulated differently. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.5393 mL | 7.6965 mL | 15.3929 mL | |
| 5 mM | 0.3079 mL | 1.5393 mL | 3.0786 mL | |
| 10 mM | 0.1539 mL | 0.7696 mL | 1.5393 mL |