Physicochemical Properties
| Molecular Formula | C21H20CLN5O |
| Molecular Weight | 393.87 |
| Exact Mass | 393.135 |
| Elemental Analysis | C, 64.04; H, 5.12; Cl, 9.00; N, 17.78; O, 4.06 |
| CAS # | 1637300-25-4 |
| Related CAS # | 1637300-25-4 |
| PubChem CID | 125286314 |
| Appearance | Solid powder |
| Density | 1.3±0.1 g/cm3 |
| Index of Refraction | 1.661 |
| LogP | 4.65 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 6 |
| Heavy Atom Count | 28 |
| Complexity | 489 |
| Defined Atom Stereocenter Count | 0 |
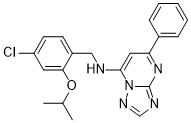
| SMILES | ClC1C=CC(=C(C=1)OC(C)C)CNC1=CC(C2C=CC=CC=2)=NC2=NC=NN12 |
| InChi Key | WBYNZQXAAWPAGR-UHFFFAOYSA-N |
| InChi Code | InChI=1S/C21H20ClN5O/c1-14(2)28-19-10-17(22)9-8-16(19)12-23-20-11-18(15-6-4-3-5-7-15)26-21-24-13-25-27(20)21/h3-11,13-14,23H,12H2,1-2H3 |
| Chemical Name | N-[(4-chloro-2-propan-2-yloxyphenyl)methyl]-5-phenyl-[1,2,4]triazolo[1,5-a]pyrimidin-7-amine |
| Synonyms | AF64394; AF-64394; AF64,394; 1637300-25-4; N-(4-chloro-2-isopropoxybenzyl)-5-phenyl-[1,2,4]triazolo[1,5-a]pyrimidin-7-amine; AF 64,394; (4-Chloro-2-isopropoxy-benzyl)-(5-phenyl-[1,2,4]triazolo[1,5-a]pyrimidin-7-yl)-amine; N-[(4-chloro-2-propan-2-yloxyphenyl)methyl]-5-phenyl-[1,2,4]triazolo[1,5-a]pyrimidin-7-amine; N-{[4-chloro-2-(propan-2-yloxy)phenyl]methyl}-5-phenyl-[1,2,4]triazolo[1,5-a]pyrimidin-7-amine; CHEMBL5415991; AF 64394 |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | GPR3 ( pIC50 = 7.3 ) |
| ln Vitro | AF64394 is an inverse agonist of GPR3, with a pIC50 of 7.3. Although AF34394 and its related compounds are relatively lipophilic and only have modest potency (e.g., AF34394 clog P=5.2, LLE=2.1, and 14b clog P=3.1 and LLE=3.2), the series exhibits encouraging surface area ratios and serves as a great starting point for additional optimization[1]. |
| Enzyme Assay | Compound potencies were determined by measuring changes in intracellular cAMP (GPR3-R coupled to Gs). Time resolved fluorescence from Cisbio (cAMP HTRF®) was applied to measure cAMP. This technique is based on a competitive immunoassay using cryptate-labeled anti-cAMP antibody and d2-labeled cAMP. A 384-well assay format with a total assay volume of 20 μL was used. 2500 HEK293-GPR3 (transfected cells GPR3 inducible cell line) cells were incubated with sample compounds for 30 min at 37 °C using DPBS containing 0.5 mM IBMX as stimulation buffer. After the addition of HTRF® detection reagents signals at 620 and 665 nm (raw counts: ratio of 665:620) were detected after a 3 h incubation. Values were calculated by nonlinear regression using the sigmoid concentration–response (variable slope) using Xlfit 4. All values reported are average of at least 4 determinations. GPR6 and 12 potencies were similarly determined. [1] |
| References |
[1]. The identification of GPR3 inverse agonist AF64394; the first small molecule inhibitor of GPR3 receptor function. Bioorg Med Chem Lett. 2014 Nov 15;24(22):5195-8. |
| Additional Infomation |
The identification of the novel and selective GPR3 inverse agonist AF64394, the first small molecule inhibitor of GPR3 receptor function, is described. Structure activity relationships and syntheses based around AF64394 are reported. [1] In summary, in inverse agonist AF64394 and related compounds we have identified the first true small molecule ligands for GPR3 and the first inhibitors of GPR3 receptor function. The compounds are specific and selective and thus form useful tools for delineating the biology of GPR3. Whilst AF34394 and related compounds are only of modest potency and are relatively lipophilic (e.g., AF34394 c log P = 5.2, LLE19 = 2.1, and 14b c log P 3.1 and LLE 3.2) the series shows promising SAR and represents an excellent start point for further optimisation. [1] |
Solubility Data
| Solubility (In Vitro) | DMSO: ≥ 125 mg/mL (~317.4 mM) |
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.08 mg/mL (5.28 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 20.8 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 2.08 mg/mL (5.28 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 20.8 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.5389 mL | 12.6945 mL | 25.3891 mL | |
| 5 mM | 0.5078 mL | 2.5389 mL | 5.0778 mL | |
| 10 mM | 0.2539 mL | 1.2695 mL | 2.5389 mL |