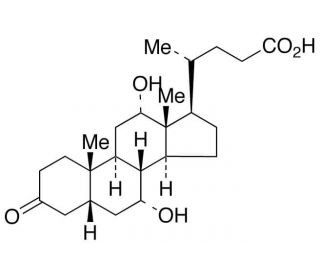
3-Oxocholic acid is an oxo-bile acid (secondary bile acid) and a metabolite of cholic acid isolated from C. perfringens, which is a bacteria found in the intestines of mammals and in the environment. 3-Oxocholic acid serum levels increase following ileal transposition surgery in rats.
Physicochemical Properties
| Molecular Formula | C₂₄H₃₈O₅ |
| Molecular Weight | 406.56 |
| Exact Mass | 406.272 |
| CAS # | 2304-89-4 |
| PubChem CID | 5283956 |
| Appearance | White to off-white solid powder |
| Density | 1.180±0.06g/ml(Predicted) |
| Boiling Point | 583.0±50.0℃(Predicted) |
| Melting Point | 185℃ |
| Vapour Pressure | 5.08E-16mmHg at 25°C |
| LogP | 3.656 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 4 |
| Heavy Atom Count | 29 |
| Complexity | 676 |
| Defined Atom Stereocenter Count | 10 |
| SMILES | C[C@H](CCC(=O)O)[C@H]1CC[C@@H]2[C@@]1([C@H](C[C@H]3[C@H]2[C@@H](C[C@H]4[C@@]3(CCC(=O)C4)C)O)O)C |
| InChi Key | OEKUSRBIIZNLHZ-DJDNIQJZSA-N |
| InChi Code | InChI=1S/C24H38O5/c1-13(4-7-21(28)29)16-5-6-17-22-18(12-20(27)24(16,17)3)23(2)9-8-15(25)10-14(23)11-19(22)26/h13-14,16-20,22,26-27H,4-12H2,1-3H3,(H,28,29)/t13-,14+,16-,17+,18+,19-,20+,22+,23+,24-/m1/s1 |
| Chemical Name | (4R)-4-[(5R,7R,8R,9S,10S,12S,13R,14S,17R)-7,12-dihydroxy-10,13-dimethyl-3-oxo-1,2,4,5,6,7,8,9,11,12,14,15,16,17-tetradecahydrocyclopenta[a]phenanthren-17-yl]pentanoic acid |
| Synonyms | 3Oxocholic acid 3 Oxocholic acid |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| ln Vivo | Ileal transposition (IT) surgery is performed on male Goto-Kakizaki (GK) rats. The metabolomics research shows that the IT rats have higher 3-Oxocholic acid than the Sham-IT animals[2]. |
| References |
[1]. Identification of 7alpha-, 12alpha-dihydroxy-3-oxo cholanoic acid as the major degradation product from cholic by C. perfringens. J Steroid Biochem. 1978 Apr;9(4):353-8. [2]. The Changes of Serum Metabolites in Diabetic GK Rats after Ileal Transposition Surgery. Obes Surg. 2019 Mar;29(3):882-890. [3]. Intestinal Crosstalk between Bile Acids and Microbiota and Its Impact on Host Metabolism. Cell Metab. 2016 Jul 12;24(1):41-50. |
| Additional Infomation | 7alpha,12alpha-dihydroxy-3-oxo-5beta-cholan-24-oic acid is a 3-oxo steroid that is cholic acid in which the hydroxy group at position 3 has undergone formal oxidation to the corresponding ketone. It has a role as a human metabolite. It is a bile acid, a 12alpha-hydroxy steroid, a dihydroxy-5beta-cholanic acid, a 7alpha-hydroxy steroid and a 3-oxo-5beta-steroid. It is a conjugate acid of a 7alpha,12alpha-dihydroxy-3-oxo-5beta-cholan-24-oate. |
Solubility Data
| Solubility (In Vitro) | DMSO : ~100 mg/mL (~245.97 mM) |
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.5 mg/mL (6.15 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 2: ≥ 2.5 mg/mL (6.15 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.4597 mL | 12.2983 mL | 24.5966 mL | |
| 5 mM | 0.4919 mL | 2.4597 mL | 4.9193 mL | |
| 10 mM | 0.2460 mL | 1.2298 mL | 2.4597 mL |