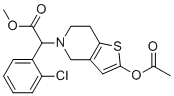
Vicagrel is the acetated form of Clopidogrel with antithrombotic activity. It treats thrombosis by acting as a P2Y12 platelet inhibitor. Vicagrel had antiplatelet potency that was significantly higher than clopidogrel and comparable to prasugrel. According to preliminary pharmacokinetic study results, clopidogrel thiolactone generated from vicagrel had a 6-fold higher bioavailability than clopidogrel, suggesting a much lower clinically effective dose for vicagrel when compared to clopidogrel. Overall, vicagrel is a more promising and safer antiplatelet agent than clopidogrel, with potential benefits that include: (1) no drug resistance for CYP2C19 poor metabolizers; (2) lower dose-related toxicity because of a much lower effective dose; and (3) a faster onset of action.
Physicochemical Properties
| Molecular Formula | C18H18CLNO4S | |
| Molecular Weight | 379.86 | |
| Exact Mass | 379.065 | |
| Elemental Analysis | C, 56.92; H, 4.78; Cl, 9.33; N, 3.69; O, 16.85; S, 8.44 | |
| CAS # | 1314081-53-2 | |
| Related CAS # |
|
|
| PubChem CID | 53378151 | |
| Appearance | White to off-white solid powder | |
| LogP | 3.537 | |
| Hydrogen Bond Donor Count | 0 | |
| Hydrogen Bond Acceptor Count | 6 | |
| Rotatable Bond Count | 6 | |
| Heavy Atom Count | 25 | |
| Complexity | 506 | |
| Defined Atom Stereocenter Count | 1 | |
| SMILES | ClC1C=CC=CC=1[C@@H](C(=O)OC)N1CC2C=C(OC(C)=O)SC=2CC1 |
|
| InChi Key | GNHHCBSBCDGWND-KRWDZBQOSA-N | |
| InChi Code | InChI=1S/C18H18ClNO4S/c1-11(21)24-16-9-12-10-20(8-7-15(12)25-16)17(18(22)23-2)13-5-3-4-6-14(13)19/h3-6,9,17H,7-8,10H2,1-2H3/t17-/m0/s1 | |
| Chemical Name | methyl (2S)-2-(2-acetyloxy-6,7-dihydro-4H-thieno[3,2-c]pyridin-5-yl)-2-(2-chlorophenyl)acetate | |
| Synonyms |
|
|
| HS Tariff Code | 2934.99.9001 | |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| ln Vitro | Vicagrel (compound 9a) (20 μM, 30 min) stimulates the in vitro rat liver microsomal production of the active metabolite[1]. |
| ln Vivo |
Vicagrel (compound 9a) (oral, 3 mg/kg) inhibits the aggregation of platelets in rats induced by ADP[1]. Vicagrel (orally, 1.14 mg/mL, 0–24 h) has a low clinically effective dose, good bioavailability, and good preliminary pharmacokinetics[1]. Vicagrel (oral, 5 g/kg, single, for 14 days) exhibits minimal dose-related toxicity in mouse[1]. |
| Animal Protocol |
Male Wistar rats, (200−250 g) 3 mg/kg oral |
| References |
[1]. Method for preparing vicagrel. Patent. WO2014040498A1. [2]. Overcoming clopidogrel resistance: discovery of vicagrel as a highly potent and orally bioavailable antiplatelet agent. J Med Chem. 2012 Apr 12;55(7):3342-52. |
| Additional Infomation | Vicagrel is under investigation in clinical trial NCT03599284 (The Efficacy, Safety and Pharmacokinetic of Antiplatelet Therapy for Vicagrel). |
Solubility Data
| Solubility (In Vitro) |
|
|||
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.08 mg/mL (5.48 mM) (saturation unknown) in 10% DMSO + 40% PEG300 +5% Tween-80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 20.8 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 + to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.6325 mL | 13.1627 mL | 26.3255 mL | |
| 5 mM | 0.5265 mL | 2.6325 mL | 5.2651 mL | |
| 10 mM | 0.2633 mL | 1.3163 mL | 2.6325 mL |