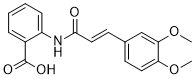
Tranilast (also known as MK 341; SB 252218; trade name Rizaben), an analog of a tryptophan metabolite, is an antiallergic drug developed by Kissei Pharmaceuticals and was approved in 1982 for use in Japan and South Korea for bronchial asthma. Indications were expanded in the 1980s to include hypertrophic and keloid scars. Interleukin-6 synthesis in endothelial cells is inhibited by tiranlast. It prevents the growth of neurofibroma cells and lowers the amount of collagen synthesized by fibroblasts. Originally discovered as an anti-allergic medication, tranilast was prescribed to treat inflammatory conditions like hypertrophic scars, atypical dermatitis, bronchial asthma, and allergic conjunctivitis. It could therefore be useful in the treatment of a variety of ailments, as the results subsequently demonstrated. Proliferative disorders, cancer, cardiovascular issues, autoimmune disorders, ocular diseases, diabetes, renal diseases, and fibrosis are just a few of the disease states in which tranilast has been shown to have positive effects.
Physicochemical Properties
| Molecular Formula | C18H17NO5 |
| Molecular Weight | 327.34 |
| Exact Mass | 327.11 |
| Elemental Analysis | C, 66.05; H, 5.23; N, 4.28; O, 24.44 |
| CAS # | 53902-12-8 |
| Related CAS # | trans-Tranilast; 70806-55-2; Tranilast sodium; 104931-56-8 |
| PubChem CID | 5282230 |
| Appearance | Light yellow to green yellow solid powder |
| Density | 1.3±0.1 g/cm3 |
| Boiling Point | 585.5±50.0 °C at 760 mmHg |
| Melting Point | 166-168ºC |
| Flash Point | 307.9±30.1 °C |
| Vapour Pressure | 0.0±1.7 mmHg at 25°C |
| Index of Refraction | 1.648 |
| LogP | 4.36 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 6 |
| Heavy Atom Count | 24 |
| Complexity | 464 |
| Defined Atom Stereocenter Count | 0 |
| SMILES | O=C(O)C1=CC=CC=C1NC(/C=C/C2=CC=C(OC)C(OC)=C2)=O |
| InChi Key | NZHGWWWHIYHZNX-CSKARUKUSA-N |
| InChi Code | InChI=1S/C18H17NO5/c1-23-15-9-7-12(11-16(15)24-2)8-10-17(20)19-14-6-4-3-5-13(14)18(21)22/h3-11H,1-2H3,(H,19,20)(H,21,22)/b10-8+ |
| Chemical Name | 2-[[(E)-3-(3,4-dimethoxyphenyl)prop-2-enoyl]amino]benzoic acid |
| Synonyms | MK-341; MK 341; MK341; SB-252218; SB 252218; SB252218; Tranilast; trans-Tranilast; brand name: Rizaben; Tranilastum; Tranpro. |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | DP2 ( IC50 = 0.1 mM ); Angiotensin II |
| ln Vitro | Tranilast is an anti-allergic medication that prevents mast cells from releasing chemicals like prostaglandins and histamine, which reduces the production of collagen by fibroblasts that are derived from keloid tissues. Trenilast (3–300 mM) inhibits the production of collagen by fibroblasts from hypertrophic and keloid scar tissue, but not by fibroblasts from healthy skin. Tranistin (30–300 mM) prevents keloid fibroblasts from releasing transforming growth factor (TGF)–beta 1, which increases keloid fibroblasts' ability to synthesize collagen.[1] Tranilast helps with hypertrophic scars and keloids, which are caused by fibroblasts that proliferate abnormally and accumulate excessive amounts of collagen. The release of PGE2, IL-1 beta, and TGF-beta 1 from human monocytes/macrophages is inhibited by troglilast.[2] Tranalilast suppresses the migration that is induced by platelet-derived growth factor-BB (PDGF-BB), TGF-beta1, fetal bovine serum (FBS), and PDGF-BB. The synthesis of collagen and glycosaminoglycan is induced by TGF-beta 1 and spontaneous collagen synthesis is inhibited by tanninlast.[3] |
| ln Vivo | Tranilast reduces 3[H]-hydroxyproline incorporation induced by TGF-beta1 by 58% in the diabetic heart of rats. In the diabetic heart of rats, tranilast reduces phospho-Smad2 by 37% and attenuates cardiac fibrosis. [4] In the canine carotid artery, tranilast treatment completely stops the increase in chymaselike activity, lowers chymase mRNA levels by 43%, and lowers the carotid intima/media ratio by 63%.[5] |
| References |
[1]. Jpn J Pharmacol . 1992 Oct;60(2):91-6. [2]. Jpn J Pharmacol . 1992 Oct;60(2):85-90. [3]. Atherosclerosis . 1995 Dec;118(2):213-21. [4]. Cardiovasc Res . 2005 Feb 15;65(3):694-701. [5]. Circulation . 1999 Mar 2;99(8):1084-90. |
| Additional Infomation |
Tranilast is an amidobenzoic acid that is anthranilic acid in which one of the anilino hydrogens is replaced by a 3,4-dimethoxycinnamoyl group. It has a role as an anti-asthmatic drug, a nephroprotective agent, an anti-allergic agent, a calcium channel blocker, an antineoplastic agent, an aryl hydrocarbon receptor agonist and a hepatoprotective agent. It is a member of cinnamamides, a dimethoxybenzene, an amidobenzoic acid and a secondary carboxamide. It is functionally related to an anthranilic acid. Tranilast is an antiallergic drug developed by Kissei Pharmaceuticals. In 1982, it was approved in Japan and South Korea for the management of bronchial asthma. Indications for keloid and hypertrophic scar were added in 1993. It has been used for the treatment of allergic disorders such as asthma, allergic rhinitis and atopic dermatitis. Drug Indication For the treatment of bronchial asthma, keloid and hypertrophic scar, and allergic disorders such as asthma, allergic rhinitis and atopic dermatitis. |
Solubility Data
| Solubility (In Vitro) |
|
|||
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.5 mg/mL (7.64 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: 2.5 mg/mL (7.64 mM) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), suspension solution; with ultrasonication. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 3: ≥ 2.5 mg/mL (7.64 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly.. Solubility in Formulation 4: 5%DMSO+ Corn oil: 3.5mg/ml (10.69mM) Solubility in Formulation 5: 5 mg/mL (15.28 mM) in 30 % SBE-β-CD (add these co-solvents sequentially from left to right, and one by one), suspension solution; with ultrasonication. Solubility in Formulation 6: 4 mg/mL (12.22 mM) in 1.5% CMC-Na/saline water (add these co-solvents sequentially from left to right, and one by one), suspension solution; with ultrasonication. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.0549 mL | 15.2746 mL | 30.5493 mL | |
| 5 mM | 0.6110 mL | 3.0549 mL | 6.1099 mL | |
| 10 mM | 0.3055 mL | 1.5275 mL | 3.0549 mL |