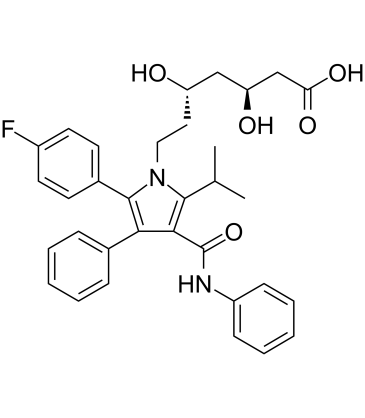
ent-Atorvastatin [(3S,5S)-Atorvastatin] is an inactive isomer/enantiomer of Atorvastatin, which is a HMGCR (HMG-CoA reductase) inhibitor and an approved blockbuster drug of the statin class of LDL cholesterol-lowering/hypolipidemic drug. ent-Atorvastatin is able to activate pregnane X receptor (PXR).
Physicochemical Properties
| Molecular Formula | C33H35FN2O5 |
| Molecular Weight | 558.65 |
| Exact Mass | 558.253 |
| CAS # | 501121-34-2 |
| Related CAS # | Atorvastatin;134523-00-5;Atorvastatin-d5 hemicalcium;222412-82-0;(3S,5S)-Atorvastatin sodium salt;1428118-38-0;(rel)-Atorvastatin;110862-48-1;Atorvastatin hemicalcium trihydrate;344920-08-7 |
| PubChem CID | 62976 |
| Appearance | Off-white to light yellow solid powder |
| Melting Point | 182-184ºC |
| LogP | 6.386 |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 12 |
| Heavy Atom Count | 41 |
| Complexity | 822 |
| Defined Atom Stereocenter Count | 2 |
| SMILES | CC(C)C1=C(C(=C(N1CC[C@@H](C[C@@H](CC(=O)O)O)O)C2=CC=C(C=C2)F)C3=CC=CC=C3)C(=O)NC4=CC=CC=C4 |
| InChi Key | XUKUURHRXDUEBC-SVBPBHIXSA-N |
| InChi Code | InChI=1S/C33H35FN2O5/c1-21(2)31-30(33(41)35-25-11-7-4-8-12-25)29(22-9-5-3-6-10-22)32(23-13-15-24(34)16-14-23)36(31)18-17-26(37)19-27(38)20-28(39)40/h3-16,21,26-27,37-38H,17-20H2,1-2H3,(H,35,41)(H,39,40)/t26-,27-/m0/s1 |
| Chemical Name | (3S,5S)-7-[2-(4-fluorophenyl)-3-phenyl-4-(phenylcarbamoyl)-5-propan-2-ylpyrrol-1-yl]-3,5-dihydroxyheptanoic acid |
| Synonyms | 3S,5S-Atorvastatin, Atorvastatin calcium trihydrate impurity E, ent-Atorvastatin |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| ln Vitro | CYP2B and CYP3A mRNA levels are increased equally by atorvastatin and its inactive enantiomer (3S,5S)-atorvastatin [1]. At a dose of 100 μM, (3S,5S)-atorvastatin causes luciferase activity, with an EC50 of 12.4 μM [2]. When 3-formylphenylboronic acid (FPBA)/l-tryptophan is added to a mixture, the intensity of l-tryptophan's fluorescence increases [3]. |
| Toxicity/Toxicokinetics |
Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation The consensus opinion is that women taking a statin should not breastfeed because of a concern with disruption of infant lipid metabolism. However, others have argued that children homozygous for familial hypercholesterolemia are treated with statins beginning at 1 year of age, that statins have low oral bioavailability, and risks to the breastfed infant are low, especially with rosuvastatin and pravastatin. Some evidence indicates that atorvastatin can be taken by nursing mothers with no obvious developmental problems in their infants. Until more data become available, an alternate drug may be preferred, especially while nursing a newborn or preterm infant. ◉ Effects in Breastfed Infants In a case series of patients with homozygous familial hypercholesterolemia, 6 patients breastfed 11 infants after restarting statin therapy postpartum. The specific statin used by these women was not reported, most of the women on statin therapy were using atorvastatin, either 40 or 80 mg, daily. Normal early child development was reported for all offspring. Children started school at the appropriate age and no learning difficulties were reported. ◉ Effects on Lactation and Breastmilk Gynecomastia has been reported in men taking atorvastatin. Serum prolactin was normal in one case where it was measured. In another case, possible rosuvastatin-induced gynecomastia resolved after the patient’s medication was changed to atorvastatin. |
| References |
[1]. Regulation of CYP2B6 and CYP3A Expression by Hydroxymethylglutaryl Coenzyme A Inhibitors in Primary Cultured Human Hepatocytes. Drug Metab Dispos. 2002 Dec;30(12):1400-5. [2]. Optical Isomers of Atorvastatin, Rosuvastatin and Fluvastatin Enantiospecifically Activate Pregnane X Receptor PXR and Induce CYP2A6, CYP2B6 and CYP3A4 in Human Hepatocytes. PLoS One. 2015 Sep 14;10(9):e0137720. [3]. High-Throughput Assay for Enantiomeric Excess Determination in 1,2- And 1,3-Diols and Direct Asymmetric Reaction Screening. Chemistry. 2017 Jul 26;23(42):10222-10229. |
Solubility Data
| Solubility (In Vitro) | DMSO : ~125 mg/mL (~223.76 mM) |
| Solubility (In Vivo) |
Note: Listed below are some common formulations that may be used to formulate products with low water solubility (e.g. < 1 mg/mL), you may test these formulations using a minute amount of products to avoid loss of samples. Injection Formulations (e.g. IP/IV/IM/SC) Injection Formulation 1: DMSO : Tween 80: Saline = 10 : 5 : 85 (i.e. 100 μL DMSO stock solution → 50 μL Tween 80 → 850 μL Saline) *Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH ₂ O to obtain a clear solution. Injection Formulation 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL DMSO → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Injection Formulation 3: DMSO : Corn oil = 10 : 90 (i.e. 100 μL DMSO → 900 μL Corn oil) Example: Take the Injection Formulation 3 (DMSO : Corn oil = 10 : 90) as an example, if 1 mL of 2.5 mg/mL working solution is to be prepared, you can take 100 μL 25 mg/mL DMSO stock solution and add to 900 μL corn oil, mix well to obtain a clear or suspension solution (2.5 mg/mL, ready for use in animals). Injection Formulation 4: DMSO : 20% SBE-β-CD in saline = 10 : 90 [i.e. 100 μL DMSO → 900 μL (20% SBE-β-CD in saline)] *Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Injection Formulation 5: 2-Hydroxypropyl-β-cyclodextrin : Saline = 50 : 50 (i.e. 500 μL 2-Hydroxypropyl-β-cyclodextrin → 500 μL Saline) Injection Formulation 6: DMSO : PEG300 : castor oil : Saline = 5 : 10 : 20 : 65 (i.e. 50 μL DMSO → 100 μLPEG300 → 200 μL castor oil → 650 μL Saline) Injection Formulation 7: Ethanol : Cremophor : Saline = 10: 10 : 80 (i.e. 100 μL Ethanol → 100 μL Cremophor → 800 μL Saline) Injection Formulation 8: Dissolve in Cremophor/Ethanol (50 : 50), then diluted by Saline Injection Formulation 9: EtOH : Corn oil = 10 : 90 (i.e. 100 μL EtOH → 900 μL Corn oil) Injection Formulation 10: EtOH : PEG300:Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL EtOH → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Oral Formulations Oral Formulation 1: Suspend in 0.5% CMC Na (carboxymethylcellulose sodium) Oral Formulation 2: Suspend in 0.5% Carboxymethyl cellulose Example: Take the Oral Formulation 1 (Suspend in 0.5% CMC Na) as an example, if 100 mL of 2.5 mg/mL working solution is to be prepared, you can first prepare 0.5% CMC Na solution by measuring 0.5 g CMC Na and dissolve it in 100 mL ddH2O to obtain a clear solution; then add 250 mg of the product to 100 mL 0.5% CMC Na solution, to make the suspension solution (2.5 mg/mL, ready for use in animals). Oral Formulation 3: Dissolved in PEG400 Oral Formulation 4: Suspend in 0.2% Carboxymethyl cellulose Oral Formulation 5: Dissolve in 0.25% Tween 80 and 0.5% Carboxymethyl cellulose Oral Formulation 6: Mixing with food powders Note: Please be aware that the above formulations are for reference only. InvivoChem strongly recommends customers to read literature methods/protocols carefully before determining which formulation you should use for in vivo studies, as different compounds have different solubility properties and have to be formulated differently. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.7900 mL | 8.9501 mL | 17.9003 mL | |
| 5 mM | 0.3580 mL | 1.7900 mL | 3.5801 mL | |
| 10 mM | 0.1790 mL | 0.8950 mL | 1.7900 mL |