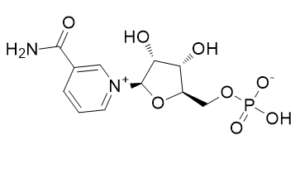
β-nicotinamide mononucleotide (also known as 'NMN', 'NAMN', and 'β-NMN') is a nucleotide intermediate derived from ribose and nicotinamide and is used in NAD+ biosynthesis produced from nicotinamide (NAM) and phosphoribosyl pyrophosphate (PRPP) by nicotinamide phosphoribosyl transferase enzyme with no toxicity. Like nicotinamide riboside , NMN is a derivative of niacin , and humans have enzymes that can use NMN to generate nicotinamide adenine dinucleotide (NADH).
Physicochemical Properties
| Molecular Formula | C11H15N2O8P |
| Molecular Weight | 334.2192 |
| Exact Mass | 334.056 |
| Elemental Analysis | C, 39.53; H, 4.52; N, 8.38; O, 38.30; P, 9.27 |
| CAS # | 1094-61-7 |
| PubChem CID | 14180 |
| Appearance | White to off-white solid powder |
| LogP | -3.38 |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 5 |
| Heavy Atom Count | 22 |
| Complexity | 455 |
| Defined Atom Stereocenter Count | 4 |
| SMILES | C1=CC(=C[N+](=C1)[C@H]2[C@@H]([C@@H]([C@H](O2)COP(=O)(O)[O-])O)O)C(=O)N |
| InChi Key | DAYLJWODMCOQEW-TURQNECASA-N |
| InChi Code | InChI=1S/C11H15N2O8P/c12-10(16)6-2-1-3-13(4-6)11-9(15)8(14)7(21-11)5-20-22(17,18)19/h1-4,7-9,11,14-15H,5H2,(H3-,12,16,17,18,19)/t7-,8-,9-,11-/m1/s1 |
| Chemical Name | ((2R,3S,4R,5R)-5-(3-carbamoylpyridin-1-ium-1-yl)-3,4-dihydroxytetrahydrofuran-2-yl)methyl hydrogen phosphate |
| Synonyms | β-nicotinamide mononucleotide; NMN; β-Nicotinamide mononucleotide; Nicotinamide Mononucleotide; beta-Nicotinamide mononucleotide; nicotinamide mononucleotide; NMN zwitterion; beta-NMN; Nicotinamide ribotide; NMN; nicotinamide nucleotide; β-NMN; β-NM; |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month Note: This product requires protection from light (avoid light exposure) during transportation and storage. |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | Endogenous Metabolite |
| ln Vitro | β-Nicotinamide mononucleotide has numerous advantageous pharmacological properties. Participation in NAD+ production is the primary mechanism by which NMN carries out its pharmacological effect. This mechanism is involved in cell biochemical processes, cardioprotection, diabetes, Alzheimer's disease, and issues connected to obesity [1]. While NAD+ levels were dramatically decreased by providing the NAD+ precursor NAM or NMN (0.5–1 mM), intracellular NAD+ levels were significantly decreased by knocking down or knocking out Nampt (KD or KO) or by treatment with the Nampt inhibitor FK866. Boost. CD8+ T cell activation and activity are inhibited by treatment with the NAD+ precursor NMN [2]. |
| ln Vivo |
Dox-induced cardiac dysfunction and mtDNA damage are prevented by β-nicotinamide mononucleotide (500 mg/kg; intraperitoneal injection; three times a week for 7–10 weeks) [3]. While Nampt metabolite β-nicotinamide mononucleotide (300 mg/kg body weight; i.p.; every two days for two weeks) greatly enhanced tumor growth in C57BL/6 mice (carrying wild-type type Hepa1-6 cells), Nampt KO considerably prevented tumor progression. shown variations in NAD+ levels in tumors treated with β-nicotinamide mononucleotide and Nampt KO [2]. In HFD-induced T2D animals, β-nicotinamide mononucleotide restores NAD+ levels, thereby mitigating glucose intolerance. Additionally, by partially activating SIRT1, β-nicotinamide mononucleotide improves hepatic insulin sensitivity and restores gene expression linked to oxidative stress, inflammatory response, and circadian rhythms [4]. NAD+ availability decreases with age and in certain disease conditions. Nicotinamide mononucleotide (NMN), a key NAD+ intermediate, has been shown to enhance NAD+ biosynthesis and ameliorate various pathologies in mouse disease models. In this study, we conducted a 12-month-long NMN administration to regular chow-fed wild-type C57BL/6N mice during their normal aging. Orally administered NMN was quickly utilized to synthesize NAD+ in tissues. Remarkably, NMN effectively mitigates age-associated physiological decline in mice. Without any obvious toxicity or deleterious effects, NMN suppressed age-associated body weight gain, enhanced energy metabolism, promoted physical activity, improved insulin sensitivity and plasma lipid profile, and ameliorated eye function and other pathophysiologies. Consistent with these phenotypes, NMN prevented age-associated gene expression changes in key metabolic organs and enhanced mitochondrial oxidative metabolism and mitonuclear protein imbalance in skeletal muscle. These effects of NMN highlight the preventive and therapeutic potential of NAD+ intermediates as effective anti-aging interventions in humans. Cell Metab. 2016 Dec 13;24(6):795-806. |
| Enzyme Assay |
Nicotinamide mononucleotide has a role as an Escherichia coli metabolite and a mouse metabolite. It is a conjugate base of a NMN(+). It is a conjugate acid of a NMN(-).
Nicotinamide ribotide is a metabolite found in or produced by Escherichia coli (strain K12, MG1655). |
| Animal Protocol |
Animal/Disease Models: C57BL6 mice (p53−/− mice) [3] Doses: 500 mg/kg Route of Administration: intraperitoneal (ip) injection; 3 times a week for 7-10 weeks Experimental Results: Protection against Dox-treated p53−/− The cardiac function of mice Dramatically diminished (weeks 7 and 10 of the study), while the doxorubicin (Dox)-induced attenuation of mitochondrial respiration and tissue ATP depletion were rescued). Type 2 diabetes (T2D) has become epidemic in our modern lifexstyle, likely due to calorie-rich diets overwhelming our adaptive metabolic pathways. One such pathway is mediated by nicotinamide phosphoribosyltransferase (NAMPT), the rate-limiting enzyme in mammalian NAD(+) biosynthesis, and the NAD(+)-dependent protein deacetylase SIRT1. Here, we show that NAMPT-mediated NAD(+) biosynthesis is severely compromised in metabolic organs by high-fat diet (HFD). Strikingly, nicotinamide mononucleotide (NMN), a product of the NAMPT reaction and a key NAD(+) intermediate, ameliorates glucose intolerance by restoring NAD(+) levels in HFD-induced T2D mice. NMN also enhances hepatic insulin sensitivity and restores gene expression related to oxidative stress, inflammatory response, and circadian rhythm, partly through SIRT1 activation. Furthermore, NAD(+) and NAMPT levels show significant decreases in multiple organs during aging, and NMN improves glucose intolerance and lipid profiles in age-induced T2D mice. These findings provide critical insights into a potential nutriceutical intervention against diet- and age-induced T2D.[4] |
| References |
[1]. Nicotinamide Mononucleotide: Exploration of Diverse Therapeutic Applications of a Potential Molecule. Biomolecules. 2019;9(1):34. Published 2019 Jan 21. [2]. NAD+ Metabolism Maintains Inducible PD-L1 Expression to Drive Tumor Immune Evasion [published online ahead of print, 2020 Nov 3]. Cell Metab. 2020;S1550-4131(20)30554-4. [3]. p53 prevents doxorubicin cardiotoxicity independently of its prototypical tumor suppressor activities. Proc Natl Acad Sci U S A. 2019;116(39):19626-19634. [4]. Yoshino J, et al Nicotinamide mononucleotide, a key NAD(+) intermediate, treats the pathophysiology of diet- and age-induced diabetes in mice. Cell Metab. 2011;14(4):528-536. |
| Additional Infomation |
NMN zwitterion is a nicotinamide mononucleotide. It has a role as an Escherichia coli metabolite and a mouse metabolite. It is a conjugate base of a NMN(+). It is a conjugate acid of a NMN(-). Nicotinamide ribotide is a metabolite found in or produced by Escherichia coli (strain K12, MG1655). Nicotinamide mononucleotide has been reported in Drosophila melanogaster, Homo sapiens, and other organisms with data available. Nicotinamide ribotide is a metabolite found in or produced by Saccharomyces cerevisiae. 3-Carbamoyl-1-beta-D-ribofuranosyl pyridinium hydroxide-5'phosphate, inner salt. A nucleotide in which the nitrogenous base, nicotinamide, is in beta-N-glycosidic linkage with the C-1 position of D-ribose. Synonyms: Nicotinamide Ribonucleotide; NMN. |
Solubility Data
| Solubility (In Vitro) | H2O : ~83.33 mg/mL (~249.33 mM) |
| Solubility (In Vivo) |
Solubility in Formulation 1: 100 mg/mL (299.20 mM) in PBS (add these co-solvents sequentially from left to right, and one by one), clear solution; with sonication. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.9920 mL | 14.9602 mL | 29.9204 mL | |
| 5 mM | 0.5984 mL | 2.9920 mL | 5.9841 mL | |
| 10 mM | 0.2992 mL | 1.4960 mL | 2.9920 mL |