Physicochemical Properties
| Molecular Formula | C6H12O6 |
| Molecular Weight | 180.16 |
| Exact Mass | 180.063 |
| CAS # | 492-61-5 |
| Related CAS # | 26874-89-5;133947-06-5 |
| PubChem CID | 64689 |
| Appearance | White to light yellow solid powder |
| Density | 1.7±0.1 g/cm3 |
| Boiling Point | 410.8±45.0 °C at 760 mmHg |
| Melting Point | 156-158ºC |
| Flash Point | 202.2±28.7 °C |
| Vapour Pressure | 0.0±2.2 mmHg at 25°C |
| Index of Refraction | 1.635 |
| LogP | -1.88 |
| Hydrogen Bond Donor Count | 5 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 1 |
| Heavy Atom Count | 12 |
| Complexity | 151 |
| Defined Atom Stereocenter Count | 5 |
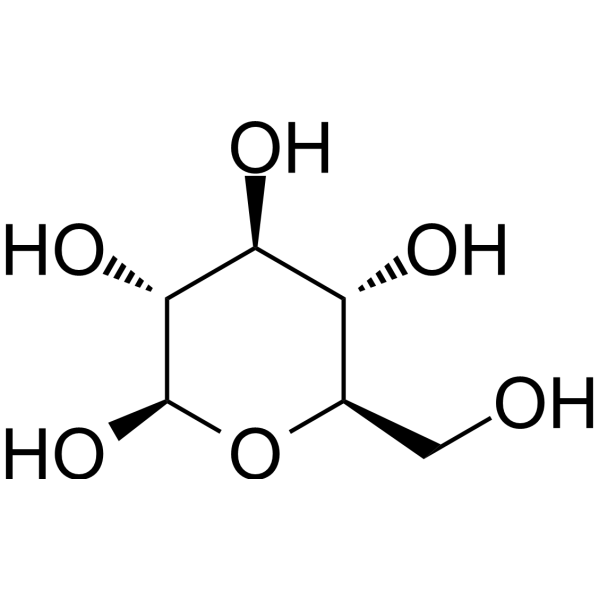
| SMILES | C([C@@H]1[C@H]([C@@H]([C@H]([C@@H](O1)O)O)O)O)O |
| InChi Key | WQZGKKKJIJFFOK-VFUOTHLCSA-N |
| InChi Code | InChI=1S/C6H12O6/c7-1-2-3(8)4(9)5(10)6(11)12-2/h2-11H,1H2/t2-,3-,4+,5-,6-/m1/s1 |
| Chemical Name | (2R,3R,4S,5S,6R)-6-(hydroxymethyl)oxane-2,3,4,5-tetrol |
| Synonyms | beta-D-glucose; beta-D-glucopyranose; 492-61-5; beta-glucose; CHEBI:15903; J4R00M814D; Beta-D-glucose anhydrous; Beta-d-glucose, anhydrous; |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month Note: (1). This product requires protection from light (avoid light exposure) during transportation and storage.(2). Please store this product in a sealed and protected environment (e.g. under nitrogen), avoid exposure to moisture. |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | Human Endogenous Metabolite |
| ln Vitro | Glucose is a monosaccharide containing six carbon atoms and an aldehyde group and is therefore referred to as an aldohexose. The glucose molecule can exist in an open-chain (acyclic) and ring (cyclic) form, the latter being the result of an intramolecular reaction between the aldehyde C atom and the C-5 hydroxyl group to form an intramolecular hemiacetal. In water solution both forms are in equilibrium and at pH 7 the cyclic one is the predominant. Glucose is a primary source of energy for living organisms. It is naturally occurring and is found in fruits and other parts of plants in its free state. In animals glucose arises from the breakdown of glycogen in a process known as glycogenolysis. Glucose is synthesized in the liver and kidneys from non-carbohydrate intermediates, such as pyruvate and glycerol, by a process known as gluconeogenesis. |
| Toxicity/Toxicokinetics |
Toxicity Summary Very high serum levels of glucose are found in untreated diabetic (type I or type II) patients. Glucose in chronic excess causes toxic effects on the structure and function of many cells and organs, including the pancreas and pancreatic islet cells. Multiple biochemical pathways and mechanisms of action for glucose toxicity have been suggested. These include glyceraldehyde auto-oxidation, protein kinase C activation, methylglyoxal formation and glycation, hexosamine metabolism, sorbitol formation, and oxidative phosphorylation. All these pathways have in common the formation of reactive oxygen species that, in excess and over time, cause chronic oxidative stress, which in turn causes defective insulin gene expression and insulin secretion as well as increased apoptosis. Exposure of endothelial cells to high glucose causes GAPDH inhibition through reactive oxygen species-activated poly(ADP-ribosyl)ation of GAPDH by poly(ADP-ribose) polymerase. Three products from glucose metabolism (glyoxal, methylglyoxal, and 3-deoxyglucosone) form advanced glycation end products (AGEs) by reacting with amino groups on intracellular and extracellular proteins. AGEs play important roles in the pathogenesis of secondary complications of diabetes, especially with regard to microvascular disease in the retina, nerves, and kidney and likely islets. Glycated hemoglobin is a particularly important AGE. A 1% increase in absolute concentrations of glycated hemoglobin is associated with about 10-20% increase in cardiovascular disease risk. Health Effects High blood glucose (>7 mM) produces the symptoms of frequent urination, increased thirst, and increased hunger. Chronic exposure to high blood glucose (i.e. untreated diabetes) can cause many complications. Acute complications include diabetic ketoacidosis (characterized by nausea, vomiting and abdominal pain, the smell of acetone on the breath) and nonketotic hyperosmolar coma. Serious long-term complications include heart disease, stroke, kidney failure, foot ulcers and damage to the eyes. The major long-term complications relate to damage to blood vessels. Diabetes doubles the risk of cardiovascular disease and about 75% of deaths in diabetics are due to coronary artery disease. Other "macrovascular" diseases are stroke, and peripheral vascular disease. The primary microvascular complications of diabetes include damage to the eyes, kidneys, and nerves. Damage to the eyes, known as diabetic retinopathy, is caused by damage to the blood vessels in the retina of the eye, and can result in gradual vision loss and potentially blindness. Damage to the kidneys, known as diabetic nephropathy, can lead to tissue scarring, urine protein loss, and eventually chronic kidney disease, sometimes requiring dialysis or kidney transplant. Damage to the nerves of the body, known as diabetic neuropathy, is the most common complication of diabetes. The symptoms can include numbness, tingling, pain, and altered pain sensation, which can lead to damage to the skin. Diabetes-related foot problems (such as diabetic foot ulcers) may occur, and can be difficult to treat, occasionally requiring amputation. Gestational diabetes can damage the health of the fetus or mother. Risks to the baby include macrosomia (high birth weight), congenital cardiac and central nervous system anomalies, and skeletal muscle malformations. Increased fetal insulin may inhibit fetal surfactant production and cause respiratory distress syndrome. Hyperbilirubinemia may result from red blood cell destruction. Adverse Effects Asthma - Reversible bronchoconstriction (narrowing of bronchioles) initiated by the inhalation of irritating or allergenic agents. Treatment Treatment involves a healthy diet, physical exercise, not using tobacco, and being a normal body weight. Blood pressure control and proper foot care are also important for people with the disease. Type 1 diabetes must be managed with insulin injections. Type 2 diabetes may be treated with medications with or without insulin. |
| References | [1]. https://pubchem.ncbi.nlm.nih.gov/compound/64689 |
| Additional Infomation |
Beta-D-glucose is d-Glucopyranose with beta configuration at the anomeric centre. It has a role as an epitope and a mouse metabolite. It is an enantiomer of a beta-L-glucose. A primary source of energy for living organisms. It is naturally occurring and is found in fruits and other parts of plants in its free state. It is used therapeutically in fluid and nutrient replacement. Glucose oxidase has been investigated for the treatment of Upper Respiratory Tract Infections. b-D-Glucose is a metabolite found in or produced by Escherichia coli (strain K12, MG1655). (2R,3R,4S,5S,6R)-6-(hydroxymethyl)oxane-2,3,4,5-tetrol has been reported in Humulus lupulus, Maclura pomifera, and other organisms with data available. Beta-D-Glucopyranose is the beta isoform of D-glucopyranose, a synthetic simple monosaccharide as an energy source. D-glucopyranose is oxidized in various tissues either under aerobic or anaerobic conditions through glycolysis, and the oxidation reaction produces carbon dioxide, water and ATP. Zymosan is an insoluble beta-1,3-glucan polysaccharide derived from, and structural component of, yeast cell walls, with potential immunostimulating activity. Upon administration, zymosan targets, binds to and activates certain Toll-like receptors, primarily TLR type 2 (TLR-2), on leukocytes and dectin-1 on macrophages. Activation of TLR2 and dectin-1 stimulates the release of pro-inflammatory mediators, and enhances innate immune responses. Beta-D-Glucose is a metabolite found in or produced by Saccharomyces cerevisiae. See also: Curdlan (annotation moved to); Zymosans (annotation moved to). |
Solubility Data
| Solubility (In Vitro) | H2O: 125 mg/mL (693.83 mM) |
| Solubility (In Vivo) |
Note: Listed below are some common formulations that may be used to formulate products with low water solubility (e.g. < 1 mg/mL), you may test these formulations using a minute amount of products to avoid loss of samples. Injection Formulations (e.g. IP/IV/IM/SC) Injection Formulation 1: DMSO : Tween 80: Saline = 10 : 5 : 85 (i.e. 100 μL DMSO stock solution → 50 μL Tween 80 → 850 μL Saline) *Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH ₂ O to obtain a clear solution. Injection Formulation 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL DMSO → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Injection Formulation 3: DMSO : Corn oil = 10 : 90 (i.e. 100 μL DMSO → 900 μL Corn oil) Example: Take the Injection Formulation 3 (DMSO : Corn oil = 10 : 90) as an example, if 1 mL of 2.5 mg/mL working solution is to be prepared, you can take 100 μL 25 mg/mL DMSO stock solution and add to 900 μL corn oil, mix well to obtain a clear or suspension solution (2.5 mg/mL, ready for use in animals). Injection Formulation 4: DMSO : 20% SBE-β-CD in saline = 10 : 90 [i.e. 100 μL DMSO → 900 μL (20% SBE-β-CD in saline)] *Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Injection Formulation 5: 2-Hydroxypropyl-β-cyclodextrin : Saline = 50 : 50 (i.e. 500 μL 2-Hydroxypropyl-β-cyclodextrin → 500 μL Saline) Injection Formulation 6: DMSO : PEG300 : castor oil : Saline = 5 : 10 : 20 : 65 (i.e. 50 μL DMSO → 100 μLPEG300 → 200 μL castor oil → 650 μL Saline) Injection Formulation 7: Ethanol : Cremophor : Saline = 10: 10 : 80 (i.e. 100 μL Ethanol → 100 μL Cremophor → 800 μL Saline) Injection Formulation 8: Dissolve in Cremophor/Ethanol (50 : 50), then diluted by Saline Injection Formulation 9: EtOH : Corn oil = 10 : 90 (i.e. 100 μL EtOH → 900 μL Corn oil) Injection Formulation 10: EtOH : PEG300:Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL EtOH → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Oral Formulations Oral Formulation 1: Suspend in 0.5% CMC Na (carboxymethylcellulose sodium) Oral Formulation 2: Suspend in 0.5% Carboxymethyl cellulose Example: Take the Oral Formulation 1 (Suspend in 0.5% CMC Na) as an example, if 100 mL of 2.5 mg/mL working solution is to be prepared, you can first prepare 0.5% CMC Na solution by measuring 0.5 g CMC Na and dissolve it in 100 mL ddH2O to obtain a clear solution; then add 250 mg of the product to 100 mL 0.5% CMC Na solution, to make the suspension solution (2.5 mg/mL, ready for use in animals). Oral Formulation 3: Dissolved in PEG400 Oral Formulation 4: Suspend in 0.2% Carboxymethyl cellulose Oral Formulation 5: Dissolve in 0.25% Tween 80 and 0.5% Carboxymethyl cellulose Oral Formulation 6: Mixing with food powders Note: Please be aware that the above formulations are for reference only. InvivoChem strongly recommends customers to read literature methods/protocols carefully before determining which formulation you should use for in vivo studies, as different compounds have different solubility properties and have to be formulated differently. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 5.5506 mL | 27.7531 mL | 55.5062 mL | |
| 5 mM | 1.1101 mL | 5.5506 mL | 11.1012 mL | |
| 10 mM | 0.5551 mL | 2.7753 mL | 5.5506 mL |