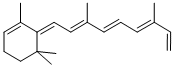
all-trans-Anhydro Retinol is a metabolite of vitamin A (Retinol) and an impurity of Vitamin A, which is widely used as an essential micronutrient and be effective in preventing xerophthalmia.
Physicochemical Properties
| Molecular Formula | C20H28 |
| Molecular Weight | 268.43632 |
| Exact Mass | 268.219 |
| CAS # | 1224-78-8 |
| PubChem CID | 5287678 |
| Appearance | Typically exists as light yellow to yellow solids at room temperature |
| Density | 0.902g/cm3 |
| Boiling Point | 378.4ºC at 760mmHg |
| Flash Point | 171.7ºC |
| Vapour Pressure | 1.37E-05mmHg at 25°C |
| Index of Refraction | 1.54 |
| LogP | 6.313 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 0 |
| Rotatable Bond Count | 4 |
| Heavy Atom Count | 20 |
| Complexity | 502 |
| Defined Atom Stereocenter Count | 0 |
| SMILES | C=C/C(=C/C=C/C(=C/C=C1\C(C)=CCCC\1(C)C)/C)/C |
| InChi Key | FWNRILWHNGFAIN-OYUWDNMLSA-N |
| InChi Code | InChI=1S/C20H28/c1-7-16(2)10-8-11-17(3)13-14-19-18(4)12-9-15-20(19,5)6/h7-8,10-14H,1,9,15H2,2-6H3/b11-8+,16-10+,17-13+,19-14- |
| Chemical Name | (6E)-6-[(2E,4E,6E)-3,7-dimethylnona-2,4,6,8-tetraenylidene]-1,5,5-trimethylcyclohexene |
| Synonyms | Anhydrovitamin A; 1224-78-8; all-trans-Anhydro Retinol; Anhydroretinol; Anhydro-retinol; (6E)-6-[(2E,4E,6E)-3,7-DIMETHYLNONA-2,4,6,8-TETRAENYLIDENE]-1,5,5-TRIMETHYLCYCLOHEXENE; 235BBF3K97; all-trans-Anhydro Retinol (90%); |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month Note: Please store this product in a sealed and protected environment (e.g. under nitrogen), avoid exposure to moisture. |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | Endogenous Metabolite |
| ln Vitro | Of the several pathways by which vitamin A and its derivatives can degrade, the present study is concerned with those leading to the formation of anhydrovitamin A. Results indicate (a) anhydro formation does not occur readily with vitamin A alcohol in the absence of a strong catalyst such as hydrogen chloride, (b) the reaction proceeds at a significant rate for solutions of the acetate in both alcoholic and hydro-alcoholic systems, (c) the conversion of the acetate to the anhydro form is much more rapid in the presence of water than in its absence, and (d) formation from the acetate does not occur in ether or hydrocarbon solvent in the absence of catalytic agents. Catalytic behavior of hydrogen chloride, perchloric acid, and acetic acid were also studied. The results on experiments made with pyridine and sodium hydroxide as possible inhibitors are also presented[1]. |
| ln Vivo | Anhydrovitamin A was fed to vitamin A-deficient rats and its metabolites isolated from the livers. These consisted of two monohydroxy and one dihydroxy derivatives and their esters. None was identical with retro-vitamin A prepared chemically from vitamin A. Neither anhydrovitamin A nor its derivatives gave rise to any detectable amount of vitamin A in the liver; nor was anhydrovitamin A present as such in the liver. Similar results were obtained after subcutaneous injection of a water dispersion of anhydrovitamin A. Two compounds similar to those isolated from the liver also were found in the kidneys. The results suggested that (a) anhydrovitamin A whether fed orally or injected subcutaneously was not absorbed and stored to any measurable extent by the rat, (b) hydroxylation mechanisms were involved in the utilization of anhydrovitamin A and (c) the growth-promoting activity of anhydrovitamin A was due to one or more of its derivatives formed in vivo[2]. |
| References |
[1]. Kinetics of Formation of Anhydrovitamin A From Vitamin A Alcohol and Its Acetate. J Am Pharm Assoc Am Pharm Assoc. 1959 Mar;48(3):155-61. [2]. The Utilization of Anhydrovitamin A by the Vitamin A-deficient Rat. Biochim Biophys Acta. 1965 Jun 15;104(1):71-7. |
| Additional Infomation | Anhydrovitamin A is a sesquiterpenoid. |
Solubility Data
| Solubility (In Vitro) | DMSO : ~10 mg/mL (~37.25 mM) |
| Solubility (In Vivo) |
Note: Listed below are some common formulations that may be used to formulate products with low water solubility (e.g. < 1 mg/mL), you may test these formulations using a minute amount of products to avoid loss of samples. Injection Formulations (e.g. IP/IV/IM/SC) Injection Formulation 1: DMSO : Tween 80: Saline = 10 : 5 : 85 (i.e. 100 μL DMSO stock solution → 50 μL Tween 80 → 850 μL Saline) *Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH ₂ O to obtain a clear solution. Injection Formulation 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL DMSO → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Injection Formulation 3: DMSO : Corn oil = 10 : 90 (i.e. 100 μL DMSO → 900 μL Corn oil) Example: Take the Injection Formulation 3 (DMSO : Corn oil = 10 : 90) as an example, if 1 mL of 2.5 mg/mL working solution is to be prepared, you can take 100 μL 25 mg/mL DMSO stock solution and add to 900 μL corn oil, mix well to obtain a clear or suspension solution (2.5 mg/mL, ready for use in animals). Injection Formulation 4: DMSO : 20% SBE-β-CD in saline = 10 : 90 [i.e. 100 μL DMSO → 900 μL (20% SBE-β-CD in saline)] *Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Injection Formulation 5: 2-Hydroxypropyl-β-cyclodextrin : Saline = 50 : 50 (i.e. 500 μL 2-Hydroxypropyl-β-cyclodextrin → 500 μL Saline) Injection Formulation 6: DMSO : PEG300 : castor oil : Saline = 5 : 10 : 20 : 65 (i.e. 50 μL DMSO → 100 μLPEG300 → 200 μL castor oil → 650 μL Saline) Injection Formulation 7: Ethanol : Cremophor : Saline = 10: 10 : 80 (i.e. 100 μL Ethanol → 100 μL Cremophor → 800 μL Saline) Injection Formulation 8: Dissolve in Cremophor/Ethanol (50 : 50), then diluted by Saline Injection Formulation 9: EtOH : Corn oil = 10 : 90 (i.e. 100 μL EtOH → 900 μL Corn oil) Injection Formulation 10: EtOH : PEG300:Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL EtOH → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Oral Formulations Oral Formulation 1: Suspend in 0.5% CMC Na (carboxymethylcellulose sodium) Oral Formulation 2: Suspend in 0.5% Carboxymethyl cellulose Example: Take the Oral Formulation 1 (Suspend in 0.5% CMC Na) as an example, if 100 mL of 2.5 mg/mL working solution is to be prepared, you can first prepare 0.5% CMC Na solution by measuring 0.5 g CMC Na and dissolve it in 100 mL ddH2O to obtain a clear solution; then add 250 mg of the product to 100 mL 0.5% CMC Na solution, to make the suspension solution (2.5 mg/mL, ready for use in animals). Oral Formulation 3: Dissolved in PEG400 Oral Formulation 4: Suspend in 0.2% Carboxymethyl cellulose Oral Formulation 5: Dissolve in 0.25% Tween 80 and 0.5% Carboxymethyl cellulose Oral Formulation 6: Mixing with food powders Note: Please be aware that the above formulations are for reference only. InvivoChem strongly recommends customers to read literature methods/protocols carefully before determining which formulation you should use for in vivo studies, as different compounds have different solubility properties and have to be formulated differently. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.7252 mL | 18.6261 mL | 37.2523 mL | |
| 5 mM | 0.7450 mL | 3.7252 mL | 7.4505 mL | |
| 10 mM | 0.3725 mL | 1.8626 mL | 3.7252 mL |