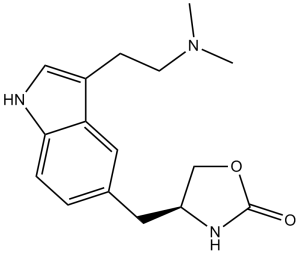
Zolmitriptan (311C90; 311 C90; trade names AscoTop, Zomig, Zomigon, Zomigoro), an approved drug for the treatment of acute migraines, is a potent and highly selective 5-HT(1B/1D) receptor agonist of the triptan class.
Physicochemical Properties
| Molecular Formula | C16H21N3O2 | |
| Molecular Weight | 287.36 | |
| Exact Mass | 287.163 | |
| Elemental Analysis | C, 66.88; H, 7.37; N, 14.62; O, 11.14 | |
| CAS # | 139264-17-8 | |
| Related CAS # |
|
|
| PubChem CID | 60857 | |
| Appearance | White to off-white solid powder | |
| Density | 1.2±0.1 g/cm3 | |
| Boiling Point | 563.3±38.0 °C at 760 mmHg | |
| Melting Point | 136-141ºC | |
| Flash Point | 294.5±26.8 °C | |
| Vapour Pressure | 0.0±1.5 mmHg at 25°C | |
| Index of Refraction | 1.620 | |
| LogP | 1.64 | |
| Hydrogen Bond Donor Count | 2 | |
| Hydrogen Bond Acceptor Count | 3 | |
| Rotatable Bond Count | 5 | |
| Heavy Atom Count | 21 | |
| Complexity | 375 | |
| Defined Atom Stereocenter Count | 1 | |
| SMILES | O1C(N([H])[C@]([H])(C1([H])[H])C([H])([H])C1C([H])=C([H])C2=C(C=1[H])C(=C([H])N2[H])C([H])([H])C([H])([H])N(C([H])([H])[H])C([H])([H])[H])=O |
|
| InChi Key | ULSDMUVEXKOYBU-ZDUSSCGKSA-N | |
| InChi Code | InChI=1S/C16H21N3O2/c1-19(2)6-5-12-9-17-15-4-3-11(8-14(12)15)7-13-10-21-16(20)18-13/h3-4,8-9,13,17H,5-7,10H2,1-2H3,(H,18,20)/t13-/m0/s1 | |
| Chemical Name | (4S)-4-[[3-[2-(dimethylamino)ethyl]-1H-indol-5-yl]methyl]-1,3-oxazolidin-2-one | |
| Synonyms | 311C 90; Flezol; zolmitriptan; 311C90; 311 C90; trade names Zomig; Zomigon; AscoTop; Zomigoro | |
| HS Tariff Code | 2934.99.9001 | |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | 5-HT1B Receptor ( IC50 = 5.01 nM ); 5-HT1D Receptor ( IC50 = 0.63 nM ); 5-HT1F Receptor ( IC50 = 63.09 nM ); Human Endogenous Metabolite | |
| ln Vitro |
|
|
| ln Vivo |
|
|
| Animal Protocol |
Anaesthetized guinea-pigs 0, 3, 10, 30 μg/kg I.v. |
|
| ADME/Pharmacokinetics |
Absorption, Distribution and Excretion Zolmitriptan tablets have a mean absolute oral bioavailability of approximately 40%, with food having no effect on the rate or extent of absorption. The dosing kinetics are linear over a range of 2.5 to 50 mg with 75% of the eventual Cmax being attained within 1 hour of dosing. The median Tmax for the tablet form is 1.5 hours, while for the orally disintegrating tablet form, it is 3 hours. The AUC across studies was in the range of 84.4-173.8 ng/mL*h while the Cmax was between 16 and 25.2 ng/mL. Zolmitriptan administered as a nasal spray is detected in the plasma within 2-5 minutes, compared to 10-15 minutes for the tablet form; the faster kinetics likely reflect fast absorption across the nasal mucosa. The bioavailability compared to the tablet is 102%, and plasma zolmitriptan concentration is maintained for 4-6 hours after intranasal delivery. The active N-desmethyl metabolite of zolmitriptan has a mean plasma concentration that is roughly two-thirds of zolmitriptan, regardless of dosage route or concentration. Zolmitriptan is primarily excreted in urine (approximately 65%) and feces (approximately 30%). Within urine, the most common form is the indole acetic acid metabolite (31%), followed by the N-oxide (7%), and N-desmethyl (4%) metabolites; the majority of zolmitriptan recovered in feces remains unchanged. Zolmitriptan has a volume of distribution between 7 and 8.4 L/kg. Zolmitriptan has a clearance of 31.5 mL/min/kg for oral tablets and 25.9 mL/min/kg for nasal administration; one-sixth of the clearance is renal. Metabolism / Metabolites Zolmitriptan is metabolized in the liver, and studies using cytochrome P450 inhibitors like [cimetidine] suggest that it is likely metabolized by CYP1A2, as well as by monoamine oxidase (MAO). Zolmitriptan metabolism results in three major metabolites: an active N-desmethyl metabolite (183C91) as well as inactive N-oxide (1652W92) and indole acetic acid (2161W92) metabolites. Zolmitriptan has known human metabolites that include Zolmitriptan N-oxide and N-Desmethylzolmitriptan. Hepatic. There have been three metabolites identified: indole acetic acid, N -oxide, and N-desmethyl metabolites. However, the N-desmethyl is the only active metabolite. Half Life: The mean elimination half-life of zolmitriptan and of the active N-desmethyl metabolite is 3 hours. Biological Half-Life Zolmitriptan has a mean elimination half-life of approximately three hours following oral or nasal administration. Its active N-desmethyl metabolite has a slightly longer (approximately 3.5 hours) half-life. |
|
| Toxicity/Toxicokinetics |
Toxicity Summary Zolmitriptan binds with high affinity to human 5-HT1B and 5-HT1D receptors leading to cranial blood vessel constriction. Current theories proposed to explain the etiology of migraine headache suggest that symptoms are due to local cranial vasodilatation and/or to the release of sensory neuropeptides (vasoactive intestinal peptide, substance P and calcitonin gene-related peptide) through nerve endings in the trigeminal system. The therapeutic activity of zolmitriptan for the treatment of migraine headache can most likely be attributed to the agonist effects at the 5HT1B/1D receptors on intracranial blood vessels (including the arterio-venous anastomoses) and sensory nerves of the trigeminal system which result in cranial vessel constriction and inhibition of pro-inflammatory neuropeptide release. Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation Preliminary evidence indicates that zolmitriptan levels in breastmilk are low. Amounts ingested by the infant are small and unlikely to affect the nursing infant, especially if the infant is older than 2 months. Concurrent use of propranolol might increase the zolmitriptan dose received by the breastfed infant substantially. Painful, burning nipples and breast pain have been reported after doses of sumatriptan and other triptans. This has occasionally been accompanied by a decrease in milk production. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk A review of four European adverse reaction databases found 26 reported cases of, painful, burning nipples, painful breasts, breast engorgement and/or painful milk ejection in women who took a triptan while nursing. Pain was sometimes intense and occasionally led to decreased milk production. Pain generally subsided with time as the drug was eliminated. The authors proposed that triptans may cause vasoconstriction of the arteries in the breast, nipples, and the arteries surrounding the alveoli and milk ducts, causing a painful sensation and a painful milk ejection reflex. Protein Binding Zolmitriptan and its active N-desmethyl metabolite remain approximately 25% bound to plasma proteins over a concentration range of 10-1000 ng/mL. |
|
| References |
[1]. Br J Pharmacol . 1997 May;121(2):157-64. [2]. Eur J Pharmacol . 2000 Jun 2;397(2-3):297-302. [3]. Eur J Pharmacol . 1998 Nov 20;361(2-3):191-7. [4]. Psychopharmacology (Berl) . 2001 Sep;157(2):131-41. |
|
| Additional Infomation |
Pharmacodynamics Zolmitriptan, like other triptans, is a serotonin (5-hydroxytryptamine; 5-HT) receptor agonist, with enhanced specificity for the 5-HT1B and 5-HT1D receptor subtypes. It is through the downstream effects of 5-HT1B/1D activation that triptans are proposed to provide acute relief of migraines. Zolmitriptan is also a vasoconstrictor, leading to possible adverse cardiovascular effects such as myocardial ischemia/infarction, arrhythmias, cerebral and subarachnoid hemorrhage, stroke, gastrointestinal ischemia, and peripheral vasospastic reactions. In addition, chest/throat/neck/jaw pain, tightness, and/or pressure has been reported, along with the possibility of medication overuse headaches and serotonin syndrome. Patients with phenylketonuria should be advised that ZOMIG-ZMT contains phenylalanine. |
Solubility Data
| Solubility (In Vitro) |
|
|||
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.5 mg/mL (8.70 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 2.5 mg/mL (8.70 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 3: 2.5 mg/mL (8.70 mM) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), suspension solution; with ultrasonication. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.4800 mL | 17.3998 mL | 34.7996 mL | |
| 5 mM | 0.6960 mL | 3.4800 mL | 6.9599 mL | |
| 10 mM | 0.3480 mL | 1.7400 mL | 3.4800 mL |