Physicochemical Properties
| Molecular Formula | C5H4N4O2 |
| Molecular Weight | 152.11 |
| Exact Mass | 152.033 |
| Elemental Analysis | C, 39.48; H, 2.65; N, 36.83; O, 21.04 |
| CAS # | 69-89-6 |
| PubChem CID | 1188 |
| Appearance | Typically exists as white to light yellow solids at room temperature |
| Density | 1.6±0.1 g/cm3 |
| Boiling Point | 834.9ºC at 760 mmHg |
| Melting Point | 300 °C |
| Flash Point | 458.7ºC |
| Index of Refraction | 1.636 |
| LogP | -0.81 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 0 |
| Heavy Atom Count | 11 |
| Complexity | 217 |
| Defined Atom Stereocenter Count | 0 |
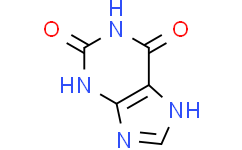
| SMILES | O=C1C2=C(N=C([H])N2[H])N([H])C(N1[H])=O |
| InChi Key | LRFVTYWOQMYALW-UHFFFAOYSA-N |
| InChi Code | InChI=1S/C5H4N4O2/c10-4-2-3(7-1-6-2)8-5(11)9-4/h1H,(H3,6,7,8,9,10,11) |
| Chemical Name | 3,7-dihydropurine-2,6-dione |
| Synonyms | xanthine; 69-89-6; 2,6-Dihydroxypurine; 2,6-dioxopurine; 1H-Purine-2,6(3H,7H)-dione; Xanthin; Xanthic oxide; Pseudoxanthine; |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | Purine base |
| ln Vitro | Caffeine and theobromine are among the many stimulants generated from Xanthine. Xanthine is the byproduct of the purine breakdown process. The enzyme Xanthine oxidase converts xanthine to uric acid. |
| ln Vivo | Caffeine is a xanthine alkaloid found in non-alcoholic beverages such as tea, coffee, and cocoa. It was discovered in tea and coffee in the 1820s, but it was not until 2000 that details of molecular events associated with caffeine biosynthesis began to be unraveled. Reviewed are the occurrence of xanthine alkaloids in the plant kingdom and the elucidation of the caffeine biosynthesis pathway, providing details of the N-methyltransferases, belonging to the motif B' methyltransferase family, which catalyze three steps in the four-step pathway leading from xanthosine to caffeine. Pathways for the metabolism and degradation of xanthine alkaloids are discussed, although as yet the genes and enzymes involved have not been isolated. This chapter also considers the in planta role of caffeine in chemical defense that has been demonstrated using transgenic caffeine-forming tobacco and chrysanthemum plants, which are resistant to attack by pathogens and herbivores. Finally, future research is considered that might lead to the production of naturally decaffeinated beverages and agricultural crops that contain elevated levels of "natural" pesticides[3]. |
| Enzyme Assay | Xanthine (3,7-dihydro-purine-2,6-dione) is generated from guanine by guanine deaminase and hypoxanthine by xanthine oxidase (XOD). The determination of xanthine in meat indicates its freshness, while its level in serum/urine provides valuable information about diagnosis and medical management of certain metabolic disorders such as xanthinuria, hyperurecemia, gout and renal failure. Although chromatographic methods such a HPLC, capillary electrophoresis and mass spectrometry are available for quantification of xanthine in biological materials, these suffer from certain limitations such as complexity, time consuming sample preparation and requirement of expensive apparatus and trained persons to operate. Immobilized XOD based biosensors have emerged as simple, rapid, sensitive and economic tools for determination of xanthine in food industries and clinical diagnosis. This review article describes the various immobilization methods of XOD and different matrices used for construction of xanthine biosensors, their classification, analytical performance and applications along with their merits and demerits. The future perspectives for improvement of present xanthine biosensors are also discussed[1]. |
| ADME/Pharmacokinetics |
Metabolism / Metabolites Xanthine is readily converted to uric acid. The enzyme xanthine oxidase makes uric acid from xanthine and hypoxanthine, which in turn are produced from other purines. In humans and higher primates, uric acid is the final oxidation (breakdown) product of purine metabolism and is excreted in urine. |
| Toxicity/Toxicokinetics |
Toxicity Summary Xanthine is a poorly soluble compound. As a result high concentrations of serum xanthine can lead to the formation of kidney stones (xanthine kidney stones) which can, over the long term, induce kidney failure. |
| References |
[1]. Pundir CS, Devi R. Biosensing methods for xanthine determination: a review. Enzyme Microb Technol. 2014;57:55-62. [2]. Central Nervous SystemStimulants. [3]. Xanthine Alkaloids: Occurrence, Biosynthesis, and Function in Plants. Prog Chem Org Nat Prod. 2017;105:1-88. |
| Additional Infomation |
9H-xanthine is an oxopurine in which the purine ring is substituted by oxo groups at positions 2 and 6 and N-9 is protonated. It has a role as a Saccharomyces cerevisiae metabolite. It is a tautomer of a 7H-xanthine. A purine base found in most body tissues and fluids, certain plants, and some urinary calculi. It is an intermediate in the degradation of adenosine monophosphate to uric acid, being formed by oxidation of hypoxanthine. The methylated xanthine compounds caffeine, theobromine, and theophylline and their derivatives are used in medicine for their bronchodilator effects. (Dorland, 28th ed) Xanthine is a metabolite found in or produced by Escherichia coli (strain K12, MG1655). Xanthine has been reported in Eleutherococcus giraldii, Drosophila melanogaster, and other organisms with data available. Xanthine is a purine base found in most body tissues and fluids, certain plants, and some urinary calculi. It is an intermediate in the degradation of adenosine monophosphate to uric acid, being formed by oxidation of hypoxanthine. The methylated xanthine compounds caffeine, theobromine, and theophylline and their derivatives are used in medicine for their bronchodilator effects. (Dorland, 28th ed.). Xanthine is a metabolite found in or produced by Saccharomyces cerevisiae. A purine base found in most body tissues and fluids, certain plants, and some urinary calculi. It is an intermediate in the degradation of adenosine monophosphate to uric acid, being formed by oxidation of hypoxanthine. The methylated xanthine compounds caffeine, theobromine, and theophylline and their derivatives are used in medicine for their bronchodilator effects. (Dorland, 28th ed) |
Solubility Data
| Solubility (In Vitro) |
1M NaOH : 8.33 mg/mL (~54.76 mM) H2O : ~5 mg/mL (~32.87 mM) DMSO : ~3.33 mg/mL (~21.89 mM) NH4OH: freely soluble |
| Solubility (In Vivo) |
Note: Listed below are some common formulations that may be used to formulate products with low water solubility (e.g. < 1 mg/mL), you may test these formulations using a minute amount of products to avoid loss of samples. Injection Formulations (e.g. IP/IV/IM/SC) Injection Formulation 1: DMSO : Tween 80: Saline = 10 : 5 : 85 (i.e. 100 μL DMSO stock solution → 50 μL Tween 80 → 850 μL Saline) *Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH ₂ O to obtain a clear solution. Injection Formulation 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL DMSO → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Injection Formulation 3: DMSO : Corn oil = 10 : 90 (i.e. 100 μL DMSO → 900 μL Corn oil) Example: Take the Injection Formulation 3 (DMSO : Corn oil = 10 : 90) as an example, if 1 mL of 2.5 mg/mL working solution is to be prepared, you can take 100 μL 25 mg/mL DMSO stock solution and add to 900 μL corn oil, mix well to obtain a clear or suspension solution (2.5 mg/mL, ready for use in animals). Injection Formulation 4: DMSO : 20% SBE-β-CD in saline = 10 : 90 [i.e. 100 μL DMSO → 900 μL (20% SBE-β-CD in saline)] *Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Injection Formulation 5: 2-Hydroxypropyl-β-cyclodextrin : Saline = 50 : 50 (i.e. 500 μL 2-Hydroxypropyl-β-cyclodextrin → 500 μL Saline) Injection Formulation 6: DMSO : PEG300 : castor oil : Saline = 5 : 10 : 20 : 65 (i.e. 50 μL DMSO → 100 μLPEG300 → 200 μL castor oil → 650 μL Saline) Injection Formulation 7: Ethanol : Cremophor : Saline = 10: 10 : 80 (i.e. 100 μL Ethanol → 100 μL Cremophor → 800 μL Saline) Injection Formulation 8: Dissolve in Cremophor/Ethanol (50 : 50), then diluted by Saline Injection Formulation 9: EtOH : Corn oil = 10 : 90 (i.e. 100 μL EtOH → 900 μL Corn oil) Injection Formulation 10: EtOH : PEG300:Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL EtOH → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Oral Formulations Oral Formulation 1: Suspend in 0.5% CMC Na (carboxymethylcellulose sodium) Oral Formulation 2: Suspend in 0.5% Carboxymethyl cellulose Example: Take the Oral Formulation 1 (Suspend in 0.5% CMC Na) as an example, if 100 mL of 2.5 mg/mL working solution is to be prepared, you can first prepare 0.5% CMC Na solution by measuring 0.5 g CMC Na and dissolve it in 100 mL ddH2O to obtain a clear solution; then add 250 mg of the product to 100 mL 0.5% CMC Na solution, to make the suspension solution (2.5 mg/mL, ready for use in animals). Oral Formulation 3: Dissolved in PEG400 Oral Formulation 4: Suspend in 0.2% Carboxymethyl cellulose Oral Formulation 5: Dissolve in 0.25% Tween 80 and 0.5% Carboxymethyl cellulose Oral Formulation 6: Mixing with food powders Note: Please be aware that the above formulations are for reference only. InvivoChem strongly recommends customers to read literature methods/protocols carefully before determining which formulation you should use for in vivo studies, as different compounds have different solubility properties and have to be formulated differently. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 6.5742 mL | 32.8709 mL | 65.7419 mL | |
| 5 mM | 1.3148 mL | 6.5742 mL | 13.1484 mL | |
| 10 mM | 0.6574 mL | 3.2871 mL | 6.5742 mL |