Physicochemical Properties
| Molecular Formula | C67H104N10O17S |
| Molecular Weight | 1353.66 |
| Exact Mass | 1352.73 |
| CAS # | 2412791-72-9 |
| PubChem CID | 118732748 |
| Appearance | Light yellow to brown ointment |
| LogP | 1.5 |
| Hydrogen Bond Donor Count | 5 |
| Hydrogen Bond Acceptor Count | 21 |
| Rotatable Bond Count | 52 |
| Heavy Atom Count | 95 |
| Complexity | 2270 |
| Defined Atom Stereocenter Count | 3 |
| SMILES | CCCC1=C(C(=O)NC(=C1)C)CNC(=O)C2=C3C=NN(C3=CC(=C2)C4=CN=C(C=C4)N5CCN(CC5)C(=O)CCOCCOCCOCCOCCOCCOCCOCCOCCOCCOCCOCCOCCNC(=O)CCCC[C@H]6[C@@H]7[C@H](CS6)NC(=O)N7)C(C)C |
| InChi Key | PSDIDGKZEPMSHC-FRQYIUQJSA-N |
| InChi Code | InChI=1S/C67H104N10O17S/c1-5-8-52-43-51(4)72-66(81)56(52)47-70-65(80)55-44-54(45-59-57(55)48-71-77(59)50(2)3)53-11-12-61(69-46-53)75-15-17-76(18-16-75)63(79)13-19-83-21-23-85-25-27-87-29-31-89-33-35-91-37-39-93-41-42-94-40-38-92-36-34-90-32-30-88-28-26-86-24-22-84-20-14-68-62(78)10-7-6-9-60-64-58(49-95-60)73-67(82)74-64/h11-12,43-46,48,50,58,60,64H,5-10,13-42,47,49H2,1-4H3,(H,68,78)(H,70,80)(H,72,81)(H2,73,74,82)/t58-,60-,64-/m0/s1 |
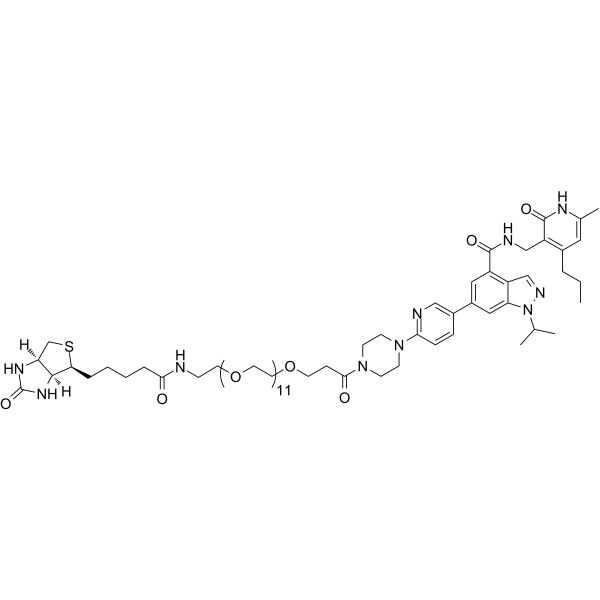
| Chemical Name | 6-[6-[4-[3-[2-[2-[2-[2-[2-[2-[2-[2-[2-[2-[2-[2-[5-[(3aS,4S,6aR)-2-oxo-1,3,3a,4,6,6a-hexahydrothieno[3,4-d]imidazol-4-yl]pentanoylamino]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]propanoyl]piperazin-1-yl]pyridin-3-yl]-N-[(6-methyl-2-oxo-4-propyl-1H-pyridin-3-yl)methyl]-1-propan-2-ylindazole-4-carboxamide |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | EZH2 17 nM (IC50) |
| ln Vitro | In the EZH2 radioactive biochemical experiment, UNC2399 (1-1000 nM) exhibits high in vitro potency (IC50=17±2 nM)[1]. UNC2399 (100 μM) enhances EZH2 in lysates from HEK293T cells[1]. |
| References |
[1]. An orally bioavailable chemical probe of the Lysine Methyltransferases EZH2 and EZH1. ACS Chem Biol. 2013; 8(6): 1324-34. [2]. Discovery of a first-in-class EZH2 selective degrader. Nat Chem Biol. 2020 Feb;16(2):214-222. |
Solubility Data
| Solubility (In Vitro) | DMSO : 100 mg/mL (73.87 mM) |
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.5 mg/mL (1.85 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 2.5 mg/mL (1.85 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 3: ≥ 2.5 mg/mL (1.85 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 0.7387 mL | 3.6937 mL | 7.3874 mL | |
| 5 mM | 0.1477 mL | 0.7387 mL | 1.4775 mL | |
| 10 mM | 0.0739 mL | 0.3694 mL | 0.7387 mL |