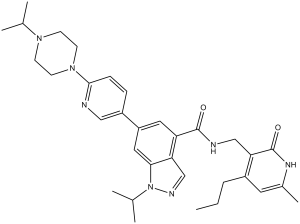
UNC1999 (UNC-1999) is an orally bioavailable, cell-permeable, selective, SAM-competitive and dual inhibitor of EZH2/EZH1 (Enhancer of zeste homolog) with antineoplastic activity. It inhibits EZH2/EZH1 with IC50s of 2 nM and 45 nM in cell-free assays, respectively, and shows >1000-fold selectivity for EZH2/EZH1 over a variety of epigenetic and non-epigenetic targets.
Physicochemical Properties
| Molecular Formula | C33H43N7O2 | |
| Molecular Weight | 569.74 | |
| Exact Mass | 569.347 | |
| CAS # | 1431612-23-5 | |
| Related CAS # |
|
|
| PubChem CID | 72551585 | |
| Appearance | Off-white to light yellow solid powder | |
| Density | 1.2±0.1 g/cm3 | |
| Boiling Point | 804.7±65.0 °C at 760 mmHg | |
| Flash Point | 440.4±34.3 °C | |
| Vapour Pressure | 0.0±2.9 mmHg at 25°C | |
| Index of Refraction | 1.643 | |
| LogP | 4.17 | |
| Hydrogen Bond Donor Count | 2 | |
| Hydrogen Bond Acceptor Count | 6 | |
| Rotatable Bond Count | 9 | |
| Heavy Atom Count | 42 | |
| Complexity | 1030 | |
| Defined Atom Stereocenter Count | 0 | |
| InChi Key | DPJNKUOXBZSZAI-UHFFFAOYSA-N | |
| InChi Code | InChI=1S/C33H43N7O2/c1-7-8-24-15-23(6)37-33(42)28(24)19-35-32(41)27-16-26(17-30-29(27)20-36-40(30)22(4)5)25-9-10-31(34-18-25)39-13-11-38(12-14-39)21(2)3/h9-10,15-18,20-22H,7-8,11-14,19H2,1-6H3,(H,35,41)(H,37,42) | |
| Chemical Name | 1-isopropyl-6-(6-(4-isopropylpiperazin-1-yl)pyridin-3-yl)-N-((6-methyl-2-oxo-4-propyl-1,2-dihydropyridin-3-yl)methyl)-1H-indazole-4-carboxamide | |
| Synonyms |
|
|
| HS Tariff Code | 2934.99.9001 | |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| ln Vitro | UNC1999 is a novel oral bioavailable inhibitor that exhibits strong in vitro potency against both wild-type and mutant EZH2. Additionally, it exhibits great potency against EZH1, a closely related H3K27 methyltransferase that shares 96% sequence identity with EZH2 in their respective catalytic domains. Over a wide spectrum of epigenetic and non-epigenetic targets, UNC1999 exhibits great selectivity for EZH2 and EZH1. It competes with the cofactor SAM but does not bind with peptide substrates. For sigma1, sigma2, histamine H3, and NET, the Ki values of UNC1999 are 4,700 nM, 65 nM, 300 nM, and 1,500 nM, in that order. DB cells, a DLBCL cell line carrying the EZH2 Y641N mutation, are specifically eliminated by NC1999. UNC1999 inhibits DB cell growth in a concentration- and time-dependent manner (EC50=633±101 nM (n=3))[1]. | ||
| ln Vivo | In male Swiss albino mice, a single intraperitoneal (IP) injection of UNC1999 at 15, 50, or 150 mg/kg produced a high Cmax (9,700-11,800 nM) and demonstrated dosage linearity. While the 15 mg/kg IP dose caused the plasma concentrations of UNC1999 to be above their cellular IC50 for about 12 hours, the 150 and 50 mg/kg IP doses caused the plasma concentrations of UNC1999 to be above their cellular IC50 for the full 24 hours. The oral bioavailability of UNC1999 was next investigated, and we are happy to report that in male Swiss albino mice, a single oral dosage of 50 mg/kg of UNC1999 produced a high Cmax (4,700 nM) and good exposure levels. After this single oral dose, UNC1999 plasma concentrations are sustained above its cellular IC50 for about 20 hours. It is noteworthy that the test animals exhibit good tolerance to all dosages, including the 150 mg/kg IP dose, and no side effects are noted [1]. | ||
| Animal Protocol |
|
||
| References |
[1]. An Orally Bioavailable Chemical Probe of the Lysine Methyltransferases EZH2 and EZH1. ACS Chem Biol. 2013;8(6):1324-34. |
||
| Additional Infomation | UNC1999 is a SAM-competitive, potent and selective inhibitor of EZH2/1. |
Solubility Data
| Solubility (In Vitro) |
|
|||
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.25 mg/mL (3.95 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 22.5 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: 2.25 mg/mL (3.95 mM) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), suspension solution; with ultrasonication. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 22.5 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 3: 2.25 mg/mL (3.95 mM) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 22.5 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.7552 mL | 8.7759 mL | 17.5519 mL | |
| 5 mM | 0.3510 mL | 1.7552 mL | 3.5104 mL | |
| 10 mM | 0.1755 mL | 0.8776 mL | 1.7552 mL |