Physicochemical Properties
| Molecular Formula | C18H25CL3N2O |
| Molecular Weight | 391.76 |
| Exact Mass | 390.103 |
| CAS # | 112465-94-8 |
| PubChem CID | 183469 |
| Appearance | Typically exists as solid at room temperature |
| Boiling Point | 496.5ºC at 760 mmHg |
| Flash Point | 254.1ºC |
| Vapour Pressure | 5.38E-10mmHg at 25°C |
| LogP | 5.212 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 3 |
| Heavy Atom Count | 24 |
| Complexity | 414 |
| Defined Atom Stereocenter Count | 2 |
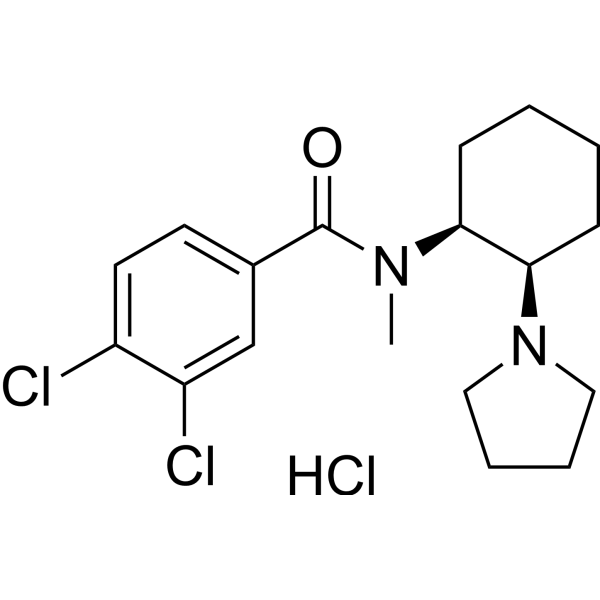
| SMILES | Cl.ClC1C=CC(C(N(C2CCCCC2N2CCCC2)C)=O)=CC=1Cl |
| InChi Key | WFUASZXAHZXJMX-PPPUBMIESA-N |
| InChi Code | InChI=1S/C18H24Cl2N2O.ClH/c1-21(18(23)13-8-9-14(19)15(20)12-13)16-6-2-3-7-17(16)22-10-4-5-11-22;/h8-9,12,16-17H,2-7,10-11H2,1H3;1H/t16-,17+;/m1./s1 |
| Chemical Name | 3,4-dichloro-N-methyl-N-[(1R,2S)-2-pyrrolidin-1-ylcyclohexyl]benzamide;hydrochloride |
| Synonyms | 112465-94-8; U-54494A HYDROCHLORIDE; U-54494ahydrochloride; cis-3,4-Dichloro-N-methyl-N-(2-(1-pyrrolidinyl)cyclohexyl)benzamide monohydrochloride; 2BZ8ZBR9LF; 3,4-dichloro-N-methyl-N-[(1R,2S)-2-pyrrolidin-1-ylcyclohexyl]benzamide;hydrochloride; U 54494A; UNII-2BZ8ZBR9LF; |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| ln Vivo | The anticonvulsant activity of (+-)-cis-3,4-dichloro-N-methyl-N-[2-(1-pyrrolidinyl)-cyclohexyl]-benz am ide monohydrochloride (U-54494A), a benzamide derivative chemically related to kappa opioid receptor agonists, was investigated in three selected seizure models of experimental epilepsy. In the maximal electroshock seizure test in mice, U-54494A (ED50 28 mg/kg i.p.) was effective, with a potency somewhat less than phenobarbital. In combination with clinically used antiepileptics, especially phenobarbital and carbamazepine, the anticonvulsant activity of the latter was significantly increased. More detailed studies with phenobarbital showed additive anticonvulsant effects. The anticonvulsant activity of U-54494A was partially antagonized by naloxone. On the other hand, this compound did not elevate the pentylenetetrazol seizure threshold (at high doses a tendency of proconvulsant action was seen). Furthermore, in unrestrained rats with chronically implanted electrodes, U-54494A (> or = 10 mg/kg) significantly reduced the duration of electrically evoked hippocampal afterdischarges. However, the focal stimulation threshold was not markedly increased. With respect to the possible mode of action, whole-cell voltage-clamp experiments on cultured neonatal rat cardiomyocytes showed that U-54494A depressed the fast sodium inward current in a concentration- and frequency-dependent manner. In summary, our results agree with earlier reports that demonstrated marked anticonvulsant effects of U-54494A in grand mal-analogous seizure tests. Moreover, in combination with some standard antiepileptics, additive effects can be found. It is suggested that, in addition to kappa opioid and excitatory amino acid receptor related effects, modulations of Na+ membrane currents may contribute to the mechanisms of action [1]. |
| Animal Protocol | 1. The effects of U-54494A and U-50488H on convulsions produced by sound have been studied in genetically epilepsy-prone DBA/2 mice and genetically epilepsy-prone rats. 2. Both compounds showed a dose-dependent anticonvulsant activity. U-54494A was less potent as an anticonvulsant than U-50488H in genetically epilepsy-prone rats and elicited a similar potency to that of U-50488H in DBA/2 mice when administered intracerebroventricularly or intraperitoneally. 3. Similar sedative and hypothermic effects were observed after the highest dose of U-54494A and U-50488H in DBA/2 mice. U-50488H seems to exhibit a greater sedative effect and to affect the rotarod test in rats much more than U-54494A. U-54494A elicited a better therapeutic index than U-50488H. 4. The anticonvulsant properties of both compounds are antagonized by high doses of naloxone and nor-binaltorphimine, a selective kappa-opioid antagonist. 5. The effects of U-50488H and U-54494A in DBA/2 mice were also antagonized by the glycine/NMDA receptor antagonist D-serine. 6. The present results suggest a possible interaction between kappa-opioid and the glycine/NMDA receptors during epileptic phenomena.[2] |
| ADME/Pharmacokinetics | U-54494A, a racemic mixture of two enantiomers, is being developed in racemic form as an anticonvulsant drug candidate. A comparative pharmacokinetic study with intravenous and oral administration of the two individual enantiomers to the dog was conducted to evaluate the potential enantioselective pharmacokinetics of U-54494. Following i.v. administration, the (-)- and (+)-enantiomers showed no significant differences in plasma clearance (0.84 +/- 0.11 versus 0.86 +/- 0.06 l/hr/kg) and terminal elimination half-life (11.2 +/- 2.7 versus 8.0 +/- 2.6 hr) for the parent drug. However, the AUC of intact drug was two-fold lower with two-fold shorter elimination half-life following the oral administration for the (+)-enantiomer as compared to the (-)-enantiomer. Higher plasma levels of the four metabolites were also observed for the (+)-than for the (-)-enantiomer, particularly after oral administration. These results suggested that the (+)-enantiomer appeared to be more extensively metabolized by first-pass effect than the (-)-enantiomer after oral dosing, and as a result, oral bioavailability for the (+)-enantiomer is only one half of that for its antipode (12.0 +/- 1.5% versus 26 +/- 9%). Enantiomer. 1996;1(2):89-96. https://pubmed.ncbi.nlm.nih.gov/9676281/ |
| References |
[1]. Anticonvulsant and related effects of U-54494A in various seizure tests. J Pharmacol Exp Ther. 1993, 267, 1. [2]. Anticonvulsant effects of U-54494A and U-50488H in genetically epilepsy-prone rats and DBA/2 mice: a possible involvement of glycine/NMDA receptor complex. Gen Pharmacol. 1993, 24, 2. |
| Additional Infomation | See also: U 54494A (annotation moved to). |
Solubility Data
| Solubility (In Vitro) | May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples |
| Solubility (In Vivo) |
Note: Listed below are some common formulations that may be used to formulate products with low water solubility (e.g. < 1 mg/mL), you may test these formulations using a minute amount of products to avoid loss of samples. Injection Formulations (e.g. IP/IV/IM/SC) Injection Formulation 1: DMSO : Tween 80: Saline = 10 : 5 : 85 (i.e. 100 μL DMSO stock solution → 50 μL Tween 80 → 850 μL Saline) *Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH ₂ O to obtain a clear solution. Injection Formulation 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL DMSO → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Injection Formulation 3: DMSO : Corn oil = 10 : 90 (i.e. 100 μL DMSO → 900 μL Corn oil) Example: Take the Injection Formulation 3 (DMSO : Corn oil = 10 : 90) as an example, if 1 mL of 2.5 mg/mL working solution is to be prepared, you can take 100 μL 25 mg/mL DMSO stock solution and add to 900 μL corn oil, mix well to obtain a clear or suspension solution (2.5 mg/mL, ready for use in animals). Injection Formulation 4: DMSO : 20% SBE-β-CD in saline = 10 : 90 [i.e. 100 μL DMSO → 900 μL (20% SBE-β-CD in saline)] *Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Injection Formulation 5: 2-Hydroxypropyl-β-cyclodextrin : Saline = 50 : 50 (i.e. 500 μL 2-Hydroxypropyl-β-cyclodextrin → 500 μL Saline) Injection Formulation 6: DMSO : PEG300 : castor oil : Saline = 5 : 10 : 20 : 65 (i.e. 50 μL DMSO → 100 μLPEG300 → 200 μL castor oil → 650 μL Saline) Injection Formulation 7: Ethanol : Cremophor : Saline = 10: 10 : 80 (i.e. 100 μL Ethanol → 100 μL Cremophor → 800 μL Saline) Injection Formulation 8: Dissolve in Cremophor/Ethanol (50 : 50), then diluted by Saline Injection Formulation 9: EtOH : Corn oil = 10 : 90 (i.e. 100 μL EtOH → 900 μL Corn oil) Injection Formulation 10: EtOH : PEG300:Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL EtOH → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Oral Formulations Oral Formulation 1: Suspend in 0.5% CMC Na (carboxymethylcellulose sodium) Oral Formulation 2: Suspend in 0.5% Carboxymethyl cellulose Example: Take the Oral Formulation 1 (Suspend in 0.5% CMC Na) as an example, if 100 mL of 2.5 mg/mL working solution is to be prepared, you can first prepare 0.5% CMC Na solution by measuring 0.5 g CMC Na and dissolve it in 100 mL ddH2O to obtain a clear solution; then add 250 mg of the product to 100 mL 0.5% CMC Na solution, to make the suspension solution (2.5 mg/mL, ready for use in animals). Oral Formulation 3: Dissolved in PEG400 Oral Formulation 4: Suspend in 0.2% Carboxymethyl cellulose Oral Formulation 5: Dissolve in 0.25% Tween 80 and 0.5% Carboxymethyl cellulose Oral Formulation 6: Mixing with food powders Note: Please be aware that the above formulations are for reference only. InvivoChem strongly recommends customers to read literature methods/protocols carefully before determining which formulation you should use for in vivo studies, as different compounds have different solubility properties and have to be formulated differently. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.5526 mL | 12.7629 mL | 25.5258 mL | |
| 5 mM | 0.5105 mL | 2.5526 mL | 5.1052 mL | |
| 10 mM | 0.2553 mL | 1.2763 mL | 2.5526 mL |