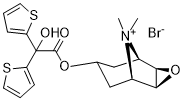
Tiotropium Bromide (BA679BR; BA-679BR; PUR-0200; PUR0200; Spiriva) is an approved anticholinergic and bronchodilator medication used as a long-acting bronchodilator for the treatment of chronic obstructive pulmonary disease (COPD).
Physicochemical Properties
| Molecular Formula | C19H22BRNO4S2 |
| Molecular Weight | 472.412 |
| Exact Mass | 471.017 |
| CAS # | 136310-93-5 |
| Related CAS # | Tiotropium-d3 bromide; 1127226-56-5; Tiotropium bromide monohydrate; 411207-31-3; Tiotropium-d6 bromide; 1126775-44-7 |
| PubChem CID | 5487426 |
| Appearance | White to off-white solid powder |
| Melting Point | 218-2200C |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 5 |
| Heavy Atom Count | 27 |
| Complexity | 564 |
| Defined Atom Stereocenter Count | 4 |
| SMILES | C[N+]1([C@@H]2CC(C[C@H]1[C@H]3[C@@H]2O3)OC(=O)C(C4=CC=CS4)(C5=CC=CS5)O)C.[Br-] |
| InChi Key | DQHNAVOVODVIMG-RGECMCKFSA-M |
| InChi Code | InChI=1S/C19H22NO4S2.BrH/c1-20(2)12-9-11(10-13(20)17-16(12)24-17)23-18(21)19(22,14-5-3-7-25-14)15-6-4-8-26-15;/h3-8,11-13,16-17,22H,9-10H2,1-2H3;1H/q+1;/p-1/t11?,12-,13+,16-,17+; |
| Chemical Name | [(1R,2R,4S,5S)-9,9-dimethyl-3-oxa-9-azoniatricyclo[3.3.1.02,4]nonan-7-yl] 2-hydroxy-2,2-dithiophen-2-ylacetate;bromide |
| Synonyms | PUR 0200; PUR0200; TIOTROPIUM BROMIDE; 136310-93-5; BA 679 BR; Spiriva Respimat; BA 679BR; BA-679 BR; BA679 BR; Tiotropium bromide [INN]; PUR-0200; BA679 BR; Tiotropium Bromide |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month Note: Please store this product in a sealed and protected environment, avoid exposure to moisture. |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | mAChR |
| ln Vitro | Tiotropium bromide causes muscle relaxation by preventing neuronal signals. Tiotropium bromide is an anticholinergic bronchodilator that acts as a long-acting, 24-hour medication that is used to treat chronic obstructive pulmonary disease. When chronic heart failure complicates chronic obstructive pulmonary disease, tiotropium bromide helps with symptoms. |
| ln Vivo |
Tiotropium bromide is a new long-lasting anticholinergic drug which, like ipratropium bromide, is a quaternary ammonium derivative. It binds with high affinity to muscarinic receptors but dissociates very slowly from M(1)- and M(3)-muscarinic receptors. Pharmacology studies have demonstrated a prolonged protective effect against cholinergic agonists and cholinergic nerve stimulation in animal and human airways. In Phase II studies single inhaled doses of tiotropium bromide have a bronchodilator and bronchoprotective effect in asthmatic and chronic obstructive pulmonary disease (COPD) patients of over 24 h. In Phase III studies, once daily inhaled tiotropium is an effective bronchodilator in COPD patients, giving great improvement in lung function and reduction in symptoms than ipratropium bromide given four times daily. The drug is well-tolerated and the only side effect of note is dryness of the mouth which occurs in approximately 10% of patients. Since, anticholinergics are the bronchodilators of choice in COPD it is likely that tiotropium bromide will become the most widely used bronchodilator for COPD patients in the future.[1] Tiotropium bromide is a quaternary ammonium compound structurally related to ipratropium and has recently been approved in the US for the long-term, once-daily, maintenance treatment of bronchospasm associated with chronic-obstructive pulmonary disease (COPD). It is available in a dry powder form, where 18 microg [corrected] of the drug is inhaled once-daily through a device, the HandiHaler). The potency and long duration of effect of this anticholinergic bronchodilator result primarily from a prolonged blockade of the M1 and M3 muscarinic receptors in the airways and a relatively more rapid dissociation from the M2 receptor (which provides inhibitory feedback). Multiple studies of up to a duration of 1 year have demonstrated its effectiveness as a bronchodilator for COPD, with a trough increase (measured approximately 24 h after administration of the drug) in forced expiratory volume in 1 s of approximately 0.12 l and a peak increase of approximately 0.25 l. Tiotropium inhalation also leads to a significant reduction in static lung volumes in hyperinflated patients with COPD; this probably contributes to the reduction in dyspnoea that is associated with long-term use of this maintenance bronchodilator. Regular use of the drug was associated with clinically meaningful increases in the Transitional Dyspnoea Index, which indicate reductions in dyspnoea associated with daily activities. Improvement in the respiratory-specific health status questionnaire, the St George's Respiratory Questionnaire component and total scores was also documented. Finally, pooled data from two 1-year studies and two 6-month studies documented 20 and 28% reductions in the number of exacerbations per patient per year. Side effects have been relatively minimal, with dry mouth the most common symptom, ranging 6 - 16% of patients and rarely leading to discontinuation of the study drug. Limited comparisons of efficacy with other bronchodilators are available. Once-daily tiotropium has been demonstrated to be clearly superior to ipratropium four times daily as a bronchodilator for COPD. Combined results from two studies comparing once-daily tiotropium to twice-daily inhalation of standard doses of salmeterol, indicate a magnitude of the bronchodilator response similar in the two drugs early in the study. However, by 6 months, the bronchodilator effect of tiotropium was somewhat greater than that of the long-acting beta-agonist. Preliminary data suggest that combining tiotropium with long-acting beta-agonists may produce additional bronchodilator action in COPD. [2] Therapy with bronchodilators forms the pharmacologic foundation of the treatment of patients with COPD. Bronchodilators can significantly lessen dyspnea, increase airflow, improve quality of life, and enhance exercise performance. While bronchodilators decrease airway resistance and lessen dynamic hyperinflation in patients with COPD, they have not been shown to alter the rate of decline in FEV1 over time, or improve patient survival. Fairly recently, a long-acting, once-daily anticholinergic medication, tiotropium bromide, has been developed which may improve symptom management in COPD patients. This paper reviews anticholinergic pharmacologic therapy for patients with COPD focusing on tiotropium bromide, and discusses treatment strategies based on disease stage. It is important to recognize that while bronchodilators improve symptoms, a multimodality treatment approach including respiratory and rehabilitative therapy, nutrition services, psychosocial counseling, and surgical care, is often necessary for the best possible care of patients with COPD.[3] Tiotropium bromide is a long-acting, once-daily inhaled anticholinergic approved for the treatment of chronic obstructive pulmonary disease (COPD). Functional and kinetic selectivity for muscarinic (M) receptors, M(1) and M(3), in the lung permit sustained bronchodilation in moderate and severe COPD. Tiotropium is associated with increased lung function, health-related quality of life and exercise tolerance, and reduced dyspnea and acute exacerbations of COPD. It has been hypothesized that tiotropium may retard the accelerated decline in lung function associated with COPD, although a recent study does not support this notion. Tiotropium is safe and well-tolerated, with few side effects. Concerns about cardiovascular side effects and increased stroke risk have been alleviated by a recent, large, multicenter, prospective, randomized trial. Herein, we discuss the pharmacology, physiology and safety profile of tiotropium, as well as the clinical studies that have demonstrated its efficacy in COPD. Additional review of airway muscarinic receptor physiology and cholinergic pathobiology relevant to COPD and asthma provides context for future experimental and therapeutic roles for tiotropium [4]. |
| Toxicity/Toxicokinetics |
Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation Although no published data exist on the use of tiotropium, its use produces negligible maternal serum levels and any drug in breastmilk would not be absorbed by the infant. The risk to the breastfed infant of maternal tiotropium inhalation is small. International guidelines agree that breastfeeding can continue during tiotropium therapy. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. |
| References |
[1]. Tiotropium bromide. Expert Opin Investig Drugs. 2001 Apr;10(4):733-40. [2]. Tiotropium bromide, a new, once-daily inhaled anticholinergic bronchodilator for chronic-obstructive pulmonary disease. Expert Opin Pharmacother. 2004 Aug;5(8):1827-35. [3]. Lipson DA. Tiotropium bromide. Int J Chron Obstruct Pulmon Dis. 2006;1(2):107-14. [4]. Tiotropium bromide for chronic obstructive pulmonary disease. Expert Rev Respir Med. 2009 Jun;3(3):211-20. [5]. The influence of treatment with formoterol, formoterol with tiotropium, formoterol with inhaled glucocorticosteroid and tiotropium on lung functions, tolerance of exercise and simple, morning everyday activities in patients with chronic obstructive pulmonary disease (COPD)]. Pneumonol Alergol Pol. 2012;80(3):255-62. |
| Additional Infomation |
Tiotropium bromide is an organic bromide salt having (1alpha,2beta,4beta,5alpha,7beta)-7-[(hydroxydi-2-thienylacetyl)oxy]-9,9-dimethyl-3-oxa-9-azoniatricyclo[3.3.1.0(2,4)]nonane as the counterion. Used (in the form of the hydrate) for maintenance treatment of airflow obstruction in patients with chronic obstructive pulmonary disease. It has a role as a bronchodilator agent and a muscarinic antagonist. It is an organic bromide salt and a quaternary ammonium salt. Tiotropium Bromide is the bromide salt form of tiotropium, a quaternary ammonium derivative of atropine and a muscarinic receptor antagonist, with bronchodilating activity. Although it does not display selectivity for specific muscarinic receptors, on topical application, tiotropium bromide acts mainly on M3 muscarinic receptors located on smooth muscle cells and submucosal glands, preventing smooth muscle contraction and mucus secretion, thus producing a bronchodilatory effect. A scopolamine derivative and CHOLINERGIC ANTAGONIST that functions as a BRONCHODILATOR AGENT. It is used in the treatment of CHRONIC OBSTRUCTIVE PULMONARY DISEASE. See also: Tiotropium (annotation moved to). Bronchodilators - long-acting b2-adrenergic agonists (formoterol and salmeterol) and a long-acting antimuscarinic drug (tiotropium), are the main drugs applied in symptomatic treatment of COPD. In patients with COPD, dyspnea is frequently associated with simple everyday activities. Two questionnaires have been published recently as a means of assessing the patients' ability to perform morning activities and symptoms. Dynamic hyperinflation is the pathophysiological disorder responsible for dyspnea and decreased exercise tolerance in COPD. Formoterol is faster than salmeterol in diminishing air-trapping. It has been shown that treatment with formoterol and tiotropium in COPD patients improves FEV(1), FVC, IC, symptoms score and quality of life in comparison with tiotropium applied alone. Among LABA and inhaled glucocorticosteroids combinations, those containing formoterol have a more beneficial effect on the ability to perform simple morning activities (budesonide/formoterol was better than fluticasone/salmeterol). Beclomethasone/formoterol - 400/24 mcg/die, in comparison with fluticasone/salmeterol - 500/100 mcg/die significantly reduced air-trapping and dyspnea in COPD patients. The comparison of budesonide/formoterol - 400/12 mcg 2 x die with beclomethasone/ /formoterol - 200/12 mcg 2 x die has shown similar influence of both combinations on FEV(1), dyspnea, 6-minute walk test, symptoms score and quality of life. The addition of budesonide and formoterol combination to tiotropium gives further benefits: reduces number of exacerbations, improves FEV1, symptoms score and performance of simple morning routines. Doctors should pay more attention to symptoms and limitations in simple activities in the morning and adequately adjust the treatment.[5] |
Solubility Data
| Solubility (In Vitro) |
DMSO: ≥ 30 mg/mL (~63.5 mM) H2O: ~25 mg/mL (~52.9 mM) |
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.08 mg/mL (4.40 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 20.8 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 2.08 mg/mL (4.40 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 20.8 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 3: ≥ 2.08 mg/mL (4.40 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 20.8 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. Solubility in Formulation 4: 27.5 mg/mL (58.21 mM) in PBS (add these co-solvents sequentially from left to right, and one by one), clear solution; with ultrasonication. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.1168 mL | 10.5840 mL | 21.1681 mL | |
| 5 mM | 0.4234 mL | 2.1168 mL | 4.2336 mL | |
| 10 mM | 0.2117 mL | 1.0584 mL | 2.1168 mL |