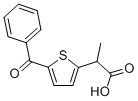
Tiaprofenic acid (Surgam, Surgamyl and Tiaprofen) is a non-steroidal anti-inflammatory drug (NSAID) approved for the treatment of pain, especially arthritic pain.
Physicochemical Properties
| Molecular Formula | C14H12O3S |
| Molecular Weight | 260.307 |
| Exact Mass | 260.051 |
| Elemental Analysis | C, 64.60; H, 4.65; O, 18.44; S, 12.32 |
| CAS # | 33005-95-7 |
| Related CAS # | Tiaprofenic acid-d3 |
| PubChem CID | 5468 |
| Appearance | Typically exists as off-white to light yellow solids at room temperature |
| Density | 1.29 g/cm3 |
| Boiling Point | 450.3ºC at 760 mmHg |
| Melting Point | 96° (isopropyl ether) |
| Flash Point | 226.1ºC |
| Index of Refraction | 1.612 |
| LogP | 3.167 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 4 |
| Heavy Atom Count | 18 |
| Complexity | 323 |
| Defined Atom Stereocenter Count | 0 |
| SMILES | CC(C1=CC=C(C(=O)C2=CC=CC=C2)S1)C(=O)O |
| InChi Key | GUHPRPJDBZHYCJ-UHFFFAOYSA-N |
| InChi Code | InChI=1S/C14H12O3S/c1-9(14(16)17)11-7-8-12(18-11)13(15)10-5-3-2-4-6-10/h2-9H,1H3,(H,16,17) |
| Chemical Name | 2-(5-benzoylthiophen-2-yl)propanoic acid |
| Synonyms | Surgam, Surgamyl; tiaprofenic acid; 33005-95-7; Surgam; 2-(5-benzoylthiophen-2-yl)propanoic acid; Tiaprofensaeure; Acido tiaprofenico; Acide tiaprofenique; Acidum tiaprofenicum; and Tiaprofen Tiaprofenic acid |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | NSAID/nonsteroidal anti-inflammatory agent; COX/cyclo-oxygenase |
| ln Vitro | Tiaprofenic acid is a propionic acid nonsteroidal anti-inflammatory drug (NSAID) with anti-inflammatory and analgesic potency greater than that of ibuprofen, aspirin (acetylsalicylic acid) or phenylbutazone and comparable to that of indomethacin, diclofenac and ketoprofen in most experimental animal models studied. Like other NSAIDs, tiaprofenic acid inhibits prostaglandin (PG) synthesis by suppressing cyclo-oxygenase, an enzyme which catalyses the conversion of arachidonic acid to cyclic endoperoxides and prostaglandins. In vitro data indicate marked inhibition of PGE2, PGF2α, prostacyclin and thromboxane A2 formation by tiaprofenic acid; a differential effect on the various prostaglandins was not observed. Statistically significant reductions in synovial fluid levels of PGE2 and PGF2α were demonstrated after administration of tiaprofenic acid to patients with rheumatoid arthritis, and were associated with clinical improvement. As with other NSAIDs, a number of other mediators of inflammation and the immune response, such as chemotaxis of human polymorphonuclear leucocytes, have been shown to be affected by tiaprofenic acid, and these actions may also contribute to the therapeutic effects of the drug[1]. |
| ln Vivo |
Tiofenac was given to female Lewis rats as an intravenous (IV) bolus (15 mg/kg) and as a continuous infusion (0.02 mg/min) for six hours. Thiofenac enhances sulfate clearance and significantly lowers serum sulfate concentrations. Additionally, there is a notable decrease in the amount of sulfate that the kidneys reabsorb [2]. Available data from studies of explant cultures of human osteoarthritic cartilage and in vivo animal and human studies have suggested that tiaprofenic acid (and possibly certain other NSAIDs such as diclofenac, naproxen and piroxicam) may preserve articular cartilage in patients with osteoarthritis. Among NSAIDs, tiaprofenic acid has been the most extensively evaluated for its possible effects on preventing cartilage degradation. Tiaprofenic acid inhibited proteoglycan catabolism, without affecting proteoglycan biosynthesis, in expiant cultures of human osteoarthritic cartilage, and stimulated in vitro synthesis of hyaluronic acid by human osteoarthritic and rheumatoid synoviocytes. Other pharmacodynamic data support these results; however, some studies did not demonstrate effects indicative of a protective effect on cartilage, and a recent large clinical trial in patients with osteoarthritis showed a neutral effect of tiaprofenic acid on cartilage. Indeed, any clinical benefit of the effects of tiaprofenic acid and alternative NSAIDs on cartilage remains to be clearly demonstrated. Animal studies evaluating injury to gastrointestinal mucosa indicate that tiaprofenic acid has a relatively low gastrotoxic potential compared with comparator NSAIDs. Faecal blood loss, endoscopic changes and/or gastrointestinal symptoms among healthy human volunteers receiving tiaprofenic acid (usually 600 mg/day) were significantly lower than with aspirin 1200 or 1800 mg/day, and comparable to findings with naproxen 750 to 1000 mg/day, ibuprofen 1200 mg/day and indomethacin 75 mg/day. Studies in normouricaemic patients with rheumatic diseases demonstrated that tiaprofenic acid 300mg every 12 hours significantly increased mean uric acid clearance. This effect was most pronounced during the first 4 hours after administration and may occur as a result of inhibition of uric acid uptake by the kidney vesicles[1]. |
| Animal Protocol | The objectives of the current investigation were: (1) to examine the effects of the nonsteroidal anti-inflammatory drug, tiaprofenic acid (TA), on sulfate renal reabsorption, and (2) to determine if concomitant prostaglandin E2 (PGE2) could reverse these effects. In crossover studies, female Lewis rats (n = 9) received either TA (as an intravenous (i.v.) bolus injection of 15 mg/kg and constant infusion of 0.02 mg/min) or its vehicle for 6 h. A blood sample was obtained at 5 h and urine was collected between 4 and 6 h. At a steady-state TA serum concentration of approximately 190 micrograms/ml, the PGE2 urinary excretion rate was inhibited by > 90% with no change in glomerular filtration rate (GFR), as measured by creatinine clearance. TA administration resulted in a significant decrease in serum sulfate concentrations (0.65 +/- 0.22 vs 1.1 +/- 0.15 mM, mean +/- SD, p < 0.01) and increase in sulfate clearance ratio (0.32 +/- 0.14 vs 0.13 +/- 0.06, p < 0.01) when compared to the vehicle-treated period. There was also a significant decrease in the fraction of sulfate reabsorbed by the kidneys (0.68 +/- 0.14 vs 0.87 +/- 0.06 in the vehicle-treated period, p < 0.01). In a second crossover study, rats received either TA or TA plus PGE2. PGE2 was administered as an infusion (0.1 micrograms/min) beginning 210 min after the start of the TA infusion. An additional group of rats served as controls and received both vehicles[2]. |
| ADME/Pharmacokinetics |
Absorption, Distribution and Excretion Bioavailability is 90% following oral administration. Metabolism / Metabolites Hepatic (10%). Sparingly metabolised in the liver to two inactive metabolites. Biological Half-Life 1.5-2.5 hours |
| References |
[1]. Tiaprofenic Acid. A Reappraisal of Its Pharmacological Properties and Use in the Management of Rheumatic Diseases. Drugs. 1995 Dec;50(6):1050-75. [2]. Tiaprofenic Acid Inhibits the Renal Reabsorption of Sulfate in Rats. Prostaglandins Leukot Essent Fatty Acids. 1993 Jul;49(1):503-8. |
| Additional Infomation |
Tiaprofenic acid is an aromatic ketone that is thiophene substituted at C-2 by benzoyl and at C-4 by a 1-carboxyethyl group. It has a role as a non-steroidal anti-inflammatory drug and a drug allergen. It is a member of thiophenes, a monocarboxylic acid and an aromatic ketone. Tiaprofenic acid is a non-steroidal anti-inflammatory drug employed in the treatment of pain, particularly arthritis pain. It belongs to the arylpropionic acid (profen) group of nonsteroidal anti-inflammatory drugs (NSAIDs). Drug Indication Tiaprofenic acid is used to treat pain, especially arthritic pain. Mechanism of Action Tiaprofenic acid belongs to a group of medicines called non-steroidal anti-inflammatory drugs (NSAIDs). It works by blocking the production of a chemical (prostaglandin) which the body produces in response to injury or certain diseases. This prostaglandin would otherwise go on to cause swelling, pain and inflammation. Pharmacodynamics Tiaprofenic acid is a non-steroidal anti-inflammatory drug of the arylpropionic acid (profen) class, used to treat pain, especially arthritic pain. The typical adult dose is 300mg twice daily. This drug is not recommended for use in the pediatric population. |
Solubility Data
| Solubility (In Vitro) | DMSO : ~100 mg/mL (~384.16 mM) |
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.5 mg/mL (9.60 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 2.5 mg/mL (9.60 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 3: ≥ 2.5 mg/mL (9.60 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.8416 mL | 19.2079 mL | 38.4157 mL | |
| 5 mM | 0.7683 mL | 3.8416 mL | 7.6831 mL | |
| 10 mM | 0.3842 mL | 1.9208 mL | 3.8416 mL |